Hypoglycemia limits the efficacy of intensive insulin therapy, especially in patients with great glucose variability. The extent to which continuous subcutaneous insulin infusion (CSII) overcomes this limitation is unclear. Our aim was to determine whether CSII is helpful for decreasing glucose variability and hypoglycemia, mainly in patients with the greatest variability.
MethodTwenty-four patients with type 1 diabetes wore a continuous glucose monitoring system sensor for 3 days before starting therapy with CSII and 6 months later. Glucose variability (SD, MAGE, M) and hypoglycemia duration (area under the curve (AUC) <70mg/dL) were compared in all patients and in those with the greatest MAGE (highest quartile).
ResultsAt 6 months, a decreased glucose variability was seen, as measured by MAGE, M, and SD (median: −28mg/dL (interquartile range, −48 to 1), p=0.03; −22 (−40 to 0), p=0.04; −11 (−23 to 0), p=0.009; respectively). Patients with the greatest initial glucose variability (MAGE quartile 4) showed a greater decrease in both MAGE (−47mg/dL (−103 to −34) vs −20 (−36 to 17), p=0.01) and AUC <70 (−10.7mg/dL×day (−15 to 0) vs −1.1 (−4.7 to 3.8), p=0.03) as compared to all others. Patients with longer initial hypoglycemia (AUC quartile 4) achieved a greater reduction in AUC <70 (−9.7mg/dL×day (−15 to −6.5) vs −0.08 (−2.9 to 3.8), p=0.003). A correlation was found between ΔMAGE–ΔAUC (r 0.4, p=0.03).
ConclusionsDuring CSII, glucose variability significantly decreased, especially in patients with the greatest initial variability. Hypoglycemia was also markedly less in patients with greater variability, with the greatest reduction occurring in those who experienced more marked hypoglycemia with CSII.
La hipoglucemia limita la eficacia de la terapia insulínica intensiva, principalmente en pacientes con gran variabilidad glucémica. Nuestro objetivo fue determinar si la terapia con infusión subcutánea contínua de insulina (ISCI) es útil y si logra disminuir la variabilidad glucémica e hipoglucemias, principalmente en los pacientes con mayor variabilidad.
MétodoSe realizó una monitorización continua de glucosa de 3 días de duración a 24 pacientes con diabetes mellitus tipo 1 (DM1) en 2 ocasiones diferentes: antes de iniciar la terapia con ISCI y 6 meses después de su implantación. Se comparó la variabilidad glucémica con distintas medidas de variabilidad (desviación estándar [SD], amplitud media de las excursiones glucémicas [MAGE], valor M) y el área bajo la curva (AUC) <70mg/dl de forma global en todos los pacientes y en aquellos con mayor variabilidad inicial (MAGE en mayor cuartil).
ResultadosA los 6 meses, se observó un descenso de la variabilidad glucémica medida como MAGE (mediana: −28mg/dl [rango interquartílico {RI}, −48 a 1], p=0,03); valor M (−22 [−40 a 0], p=0,04) y SD (−11[−23 a 0], p=0,009) en todos los pacientes. Los pacientes con mayor variabilidad glucémica inicial (MAGE cuartil 4) mostraron un mayor descenso de MAGE (−47mg/dl [−103 a −34] vs −20 [−36 a 17], p=0,01) y de AUC<70 (−10,7mg/dlxdía [−15 a 0] vs −1,1[−4,7 a 3,8], p=0,03), que el resto. Los pacientes con más tiempo en hipoglucemia inicial (AUC cuartil 4) lograron una mayor reducción del AUC<70 (−9,7mg/dlxdía [−15 a −6,5] vs −0,08 [−2,9 a 3,8], p=0,003]. Se halló una correlación entre ΔMAGE-ΔAUC [r 0,4, p=0,03].
ConclusionesDurante el tratamiento con ISCI, la variabilidad glucémica descendió significativamente, principalmente en aquellos pacientes con mayor variabilidad inicial. El tiempo en hipoglucemia también fue menor en aquellos con una mayor variabilidad. Los pacientes con más hipoglucemias iniciales experimentaron un mayor descenso de estas con ISCI.
The Diabetes Control and Complications Trial (DCCT) showed that the maintenance of strict glycemic control in patients with type 1 diabetes mellitus (T1DM) is essential in order to prevent or delay the occurrence of long-term complications.1 An exponential relationship has been shown between elevated blood glucose levels and an increased risk of retinopathy, neuropathy, and nephropathy.1–3 Consequently, one of the main objectives in the management of these diabetic patients is to try and maintain blood glucose levels as close to normal as possible.4 However, the maintenance of euglycemia is still limited today, and despite intensive therapy with multiple insulin doses (MIDs), the recommended glycosylated hemoglobin (HbA1c) value is not always achieved. This is particularly common in patients with wide glycemic excursions (high glycemic variability), in whom the achievement of adequate HbA1c without increasing hypoglycemic events represents a great and sometimes impossible challenge. The reason for this is that the lower the HbA1c, the greater the exponential risk of experiencing an episode of severe hypoglycemia.1 In addition, the occurrence of hypoglycemia not only represents the main barrier to the management of diabetes mellitus,5 but is also a significant cause of morbidity,6 mortality,7 and impaired quality of life in patients with T1DM. In fact, repeated hypoglycemic episodes may affect psychological coordination or interfere with the performance of certain tasks.5,8
Continuous subcutaneous insulin infusion (CSII) using external pumps is an increasingly used alternative approach for intensive insulin therapy. CSII has been shown to improve metabolic control, achieving slightly lower HbA1c levels9–11 and decreasing glycemic instability.12–15 Moreover, most clinical practice guidelines include it as an effective alternative in patients with recurrent moderate or severe hypoglycemic episodes and in those in whom adequate HbA1c cannot be achieved with MIDs.16,17
As regards the prevention of hypoglycemia, however, the various articles on this subject have reported conflicting results. Some studies suggest that treatment with CSII allows for decreasing hypoglycemic episodes and, thus, for improving glycemic stability,18,19 probably because of the possibility of more accurate adjustments in insulin infusion. By contrast, other reports have found no differences in the number of hypoglycemic episodes.20,21 Still other studies have achieved less glycemic variability and a greater decrease in HbA1c without an increased risk of hypoglycemia.14,15,22 On the other hand, a 2002 review concluded that CSII was even associated with an increased risk of hypoglycemia.23 Several published meta-analyses24,25 and a systematic review26 concluded that there were no significant differences in the number of hypoglycemic episodes in patients with T1DM treated with either MIDs (with analogs) or CSII. In addition, an increased risk of mild hypoglycemia was even reported in children on CSII.25 Finally, a pilot study27 conducted in patients with recurrent moderate to severe hypoglycemic episodes showed that treatment with CSII allowed for decreasing hypoglycemia and improving the perception of hypoglycemic episodes in this population.
The objective of our study was to assess whether treatment with CSII is helpful in decreasing glycemic variability and hypoglycemic time in patients with T1DM, particularly in those with greater glycemic variability and, thus, at greater risk and more difficult to manage. Our intention was to ascertain the patient profile that benefited most from treatment with CSII, in the belief that such information could be of potential value in clinical practice.
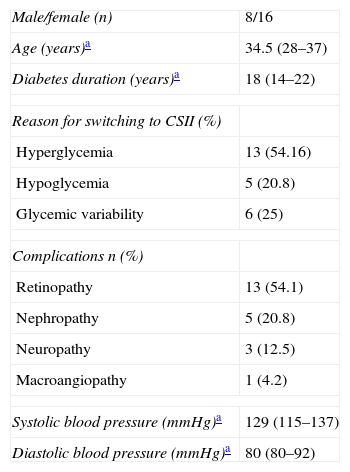
Materials and methodsA prospective, observational study was conducted at the Hospital Clínico Universitario de Santiago de Compostela. Twenty-four patients with T1DM on intensive insulin therapy were recruited. At study start, all patients were being treated with MIDs, but were about to start CSII due to hypoglycemia, hyperglycemia, or different degrees of glycemic variability. Table 1 shows the main clinical characteristics of these patients.
Baseline clinical characteristics.
| Male/female (n) | 8/16 |
| Age (years)a | 34.5 (28–37) |
| Diabetes duration (years)a | 18 (14–22) |
| Reason for switching to CSII (%) | |
| Hyperglycemia | 13 (54.16) |
| Hypoglycemia | 5 (20.8) |
| Glycemic variability | 6 (25) |
| Complications n (%) | |
| Retinopathy | 13 (54.1) |
| Nephropathy | 5 (20.8) |
| Neuropathy | 3 (12.5) |
| Macroangiopathy | 1 (4.2) |
| Systolic blood pressure (mmHg)a | 129 (115–137) |
| Diastolic blood pressure (mmHg)a | 80 (80–92) |
CSII: continuous subcutaneous insulin infusion.
Retinopathy, nephropathy, and diabetic neuropathy were respectively defined as the presence of proliferative or non-proliferative retinopathy, documented micro/macroalbuminuria, and impaired hand and/or foot sensitivity. Macrovascular complications were defined as a history of coronary and/or cerebrovascular disease. Study exclusion criteria included pregnancy, glucocorticoid treatment, skin diseases contraindicating sensor placement, febrile episodes during or in the 3 months prior to the study, psychiatric or neurological diseases preventing adequate cooperation, or T1DM duration shorter than 12 months.
Continuous subcutaneous insulin infusion devices implanted included the Minimed Paradigm® 712 and 722 (Medtronic), Accu-Check® Spirit (Roche), or Animas® (Johnson & Johnson) infusion pumps. Rapid-acting insulin analogs (lispro and aspart) were used.
ProtocolThree mandatory visits were scheduled during the study. The first visit occurred before CSII placement, the second at the time of CSII placement, and the third visit 6 months after the start of treatment with CSII. In the first and third visits, a continuous glucose monitoring (CGM) system, either the CGMS® System GoldTM or the Medtronic Guardian® REAL-TIME, was implanted at periumbilical level. This allowed for monitoring interstitial fluid glucose levels for two periods of 72 consecutive hours. The sensor was implanted on day 0, and patients returned to hospital 3 days later for sensor removal and the download of information for subsequent evaluation. The patients measured capillary blood glucose four times daily for adequate sensor calibration. In addition to these three visits, and in accordance with the “Training protocol for patients starting treatment with CSII” applicable at our hospital, frequent contacts were maintained with the diabetologist, dietician, and educational nurse in charge. This allowed for the review of different diabetes education topics such as the calculation of carbohydrate servings, catheter insertion techniques, or insulin dose adjustment.
At the baseline visit, information was collected (Table 1) on age, time since the onset of diabetes, complications, treatment, weight, body mass index (BMI), blood pressure, and HbA1c value. At the two subsequent visits, weight, blood pressure values, treatment, and any potential incidence were updated. Blood pressure was measured in the right arm after resting for 5min. The average of three consecutive measurements was used for analysis. HbA1c was measured at baseline and 6 months after the start of CSII on a venous blood sample using high-performance liquid chromatography (HPLC).
The study was approved by the ethics committee of our hospital and was conducted in accordance with Good Clinical Practice standards and the Declaration of Helsinki. All participants signed the corresponding informed consent.
Glycemic variability assessmentGlycemic variability was assessed based on data provided by CGM using three different parameters: standard deviation (SD), M-value, and mean amplitude of glycemic excursions (MAGE).
The M-value is a quantitative index of deviations of several blood glucose measurements (in a 24-h period)28,29 from an arbitrarily selected standard value (80mg/dL). It is estimated as the mean of 1000×(log plasma blood glucose/80).28
The value of MAGE is defined as the arithmetic mean of the differences between the nadir, the lowest blood glucose level, and the peak, or vice versa, when the ascending or descending peaks exceed one standard deviation from average blood glucose for the same 24-h period.30 To classify patients based on glycemic variability, baseline MAGE was divided into different quartiles (Q): very high (Q4), high (Q3), moderate (Q2), and low (Q1).
Hypoglycemia assessmentMild hypoglycemia was defined as blood glucose levels <70mg/dL. Severe hypoglycemia was defined as episodes requiring third-party assistance. The duration of hypoglycemia was determined by calculating the area under the curve (AUC) <70mg/dL by the trapezoidal method.31 Only the second monitoring day was taken into account for this calculation, in order to analyze a reliable record of 24 consecutive hours. To classify patients by hypoglycemic time, baseline AUC <70mg/dL was divided into quartiles similarly to MAGE: very high (Q4), high (Q3), moderate (Q2), and low (Q1).
Quality of lifeA validated questionnaire to assess satisfaction with the new hypoglycemic treatment (DTSQc) was administered to patients 6 months after they had started CSII. This questionnaire assessed eight items, of which six items were related to overall satisfaction with the treatment, and the remaining two items assessed the frequency of hypoglycemia or hyperglycemia in relation to prior therapy.32
Statistical analysisVariables are given as median (interquartile range). Intra-individual variations (pre-CSII vs 6 months post-CSII) in quantitative variables were calculated using a Wilcoxon test. A Mann–Whitney test was used for the comparative study of quantitative variables of two independent samples. The comparative study of more than two original categories was performed using a Jonkheere–Terpstra test. A Spearman method was performed to test for potential correlations. All of these are non-parametric tests, independent of sample distribution, and adequate for the study of a small sample such as ours. SPSS software (version 14; SPSS, Chicago, USA) was used for data analysis, a value of p<0.05 being considered statistically significant.
ResultsData from the 72-h CGM were collected from all 24 patients. Repeat CGM was not required for any patient.
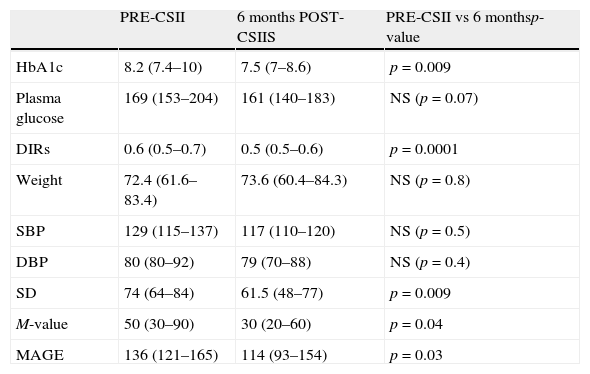
Overall effects on metabolic control, daily insulin requirements (DIRs), weight, and blood pressureAfter 6 months on CSII, HbA1c was significantly lower as compared to baseline, with a median decrease of −0.8% (interquartile range, −1.4 to 0.2%). At 6 months, a trend was seen toward a decrease in median plasma glucose (Table 2), but this did not reach statistical significance. In addition, DIRs significantly decreased at 6 months, while weight and blood pressure remained stable (Table 2).
Comparison of glycosylated hemoglobin (HbA1c, %) and plasma glucose (mg/dL) levels, daily insulin requirements (DIRs, U/kg/day), weight (kg), systolic blood pressure (SBP), diastolic blood pressure (DBP), standard deviation (SD), M-value (mg/dL), and mean amplitude of glycemic excursions (MAGE, mg/dL) before CSII (continuous subcutaneous insulin infusion) and 6 months after CSII.
| PRE-CSII | 6 months POST-CSIIS | PRE-CSII vs 6 monthsp-value | |
| HbA1c | 8.2 (7.4–10) | 7.5 (7–8.6) | p=0.009 |
| Plasma glucose | 169 (153–204) | 161 (140–183) | NS (p=0.07) |
| DIRs | 0.6 (0.5–0.7) | 0.5 (0.5–0.6) | p=0.0001 |
| Weight | 72.4 (61.6–83.4) | 73.6 (60.4–84.3) | NS (p=0.8) |
| SBP | 129 (115–137) | 117 (110–120) | NS (p=0.5) |
| DBP | 80 (80–92) | 79 (70–88) | NS (p=0.4) |
| SD | 74 (64–84) | 61.5 (48–77) | p=0.009 |
| M-value | 50 (30–90) | 30 (20–60) | p=0.04 |
| MAGE | 136 (121–165) | 114 (93–154) | p=0.03 |
Data are given as median (interquartile range); NS: not significant.
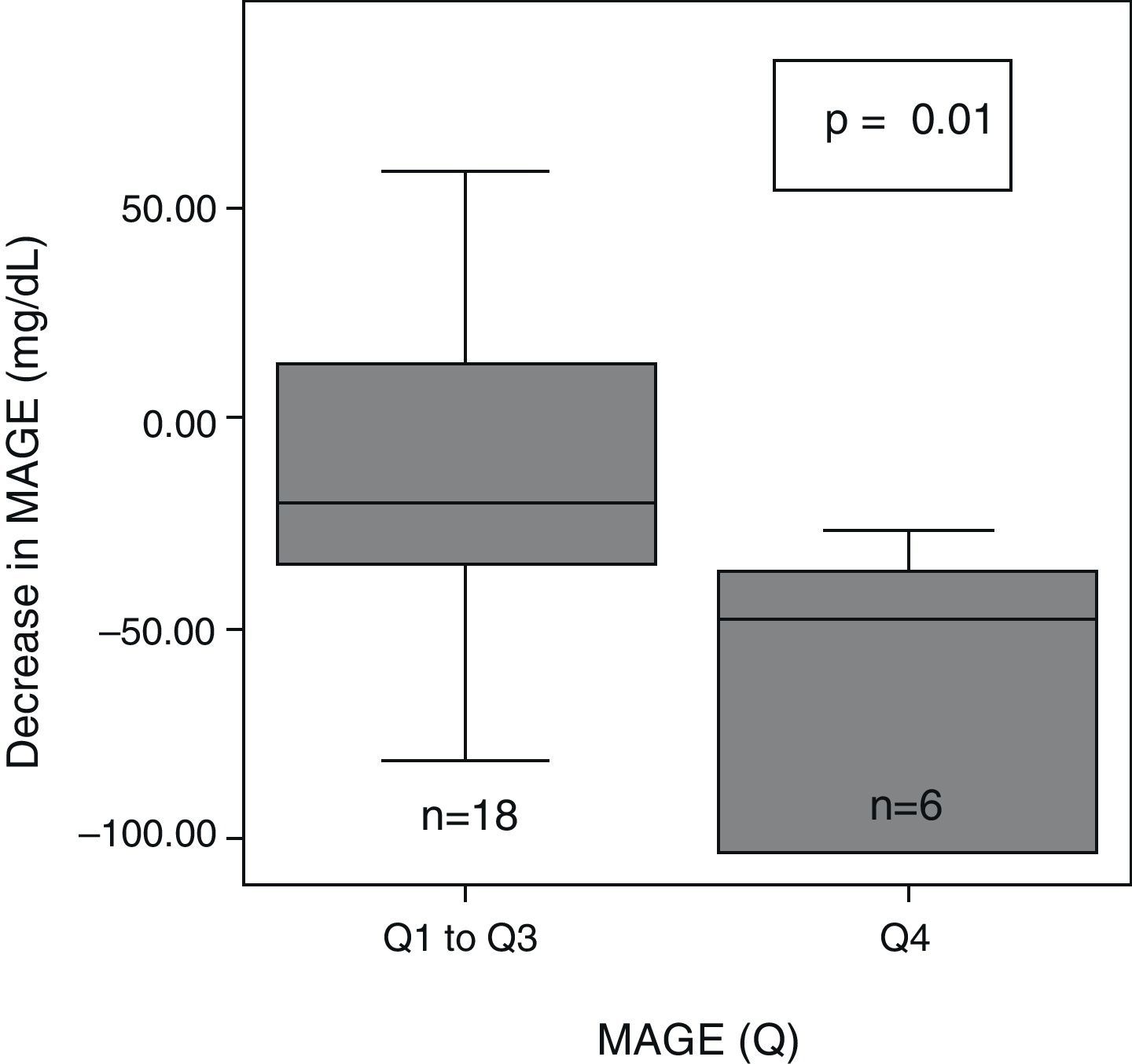
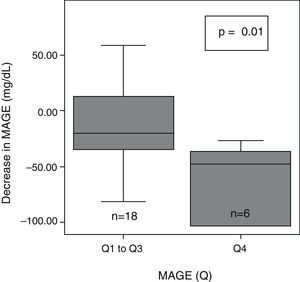
After 6 months of treatment with CSII, a significant overall decrease was seen in glycemic variability, as assessed with SD, M-value, and MAGE (Table 2). After dividing initial glycemic variability (MAGE) into four quartiles: very high (Q4: >165.9), high (Q3: 136–165.9), moderate (Q2: 121.3–136), and low (Q1: <121.3), significant differences were seen in the extent of MAGE decrease as a quartile function (p=0.02). At 6 months, MAGE decrease was greater in the patient group with higher baseline MAGE (MAGE Q4) than in all other groups (Fig. 1).
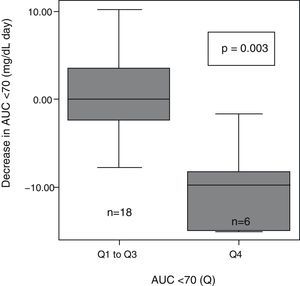
HypoglycemiaAt 6 months, an overall trend was seen toward decreased hypoglycemic time, calculated as AUC <70mg/dL (pre-CSII: median 4.1mg/dL×day (interquartile range, 0.4–8.1mg/dL×day) vs post-CSII: 1.2mg/dL/day (0–5mg/dL×day), p=0.32). A significantly greater decrease in AUC<70 (median −10mg/dL/day (interquartile range, −1.5 to 0mg/dL×day) was seen in patients with greater baseline variability (MAGE Q4) as compared to all other patients (MAGE Q1–Q3, −1.1mg/dL/day [−4.7 to 3.8mg/dL×day], p=0.04). No patient experienced severe hypoglycemia.
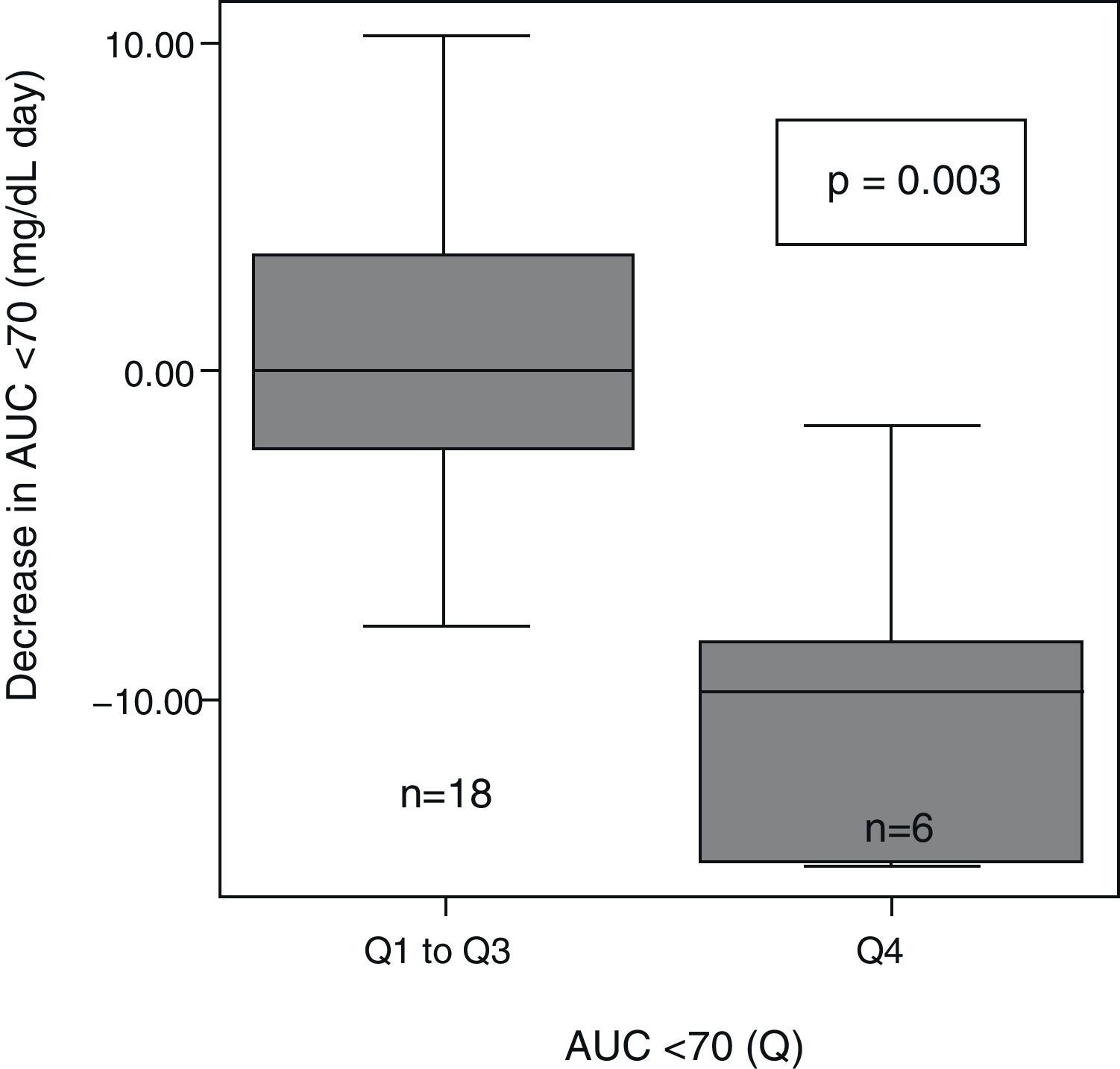
After patient classification into quartiles based on their very high (>8.1), high (8.1–4.1), moderate (4.1–0.4), and low (<0.4) baseline AUC<70mg/dL, significant differences were seen in the extent of AUC decrease between the different groups (p=0.004). AUC decrease after 6 months with CSII was greater in the patient group with higher baseline AUC<70 (Q4) as compared to all other groups (Q1–Q3) (Fig. 2).
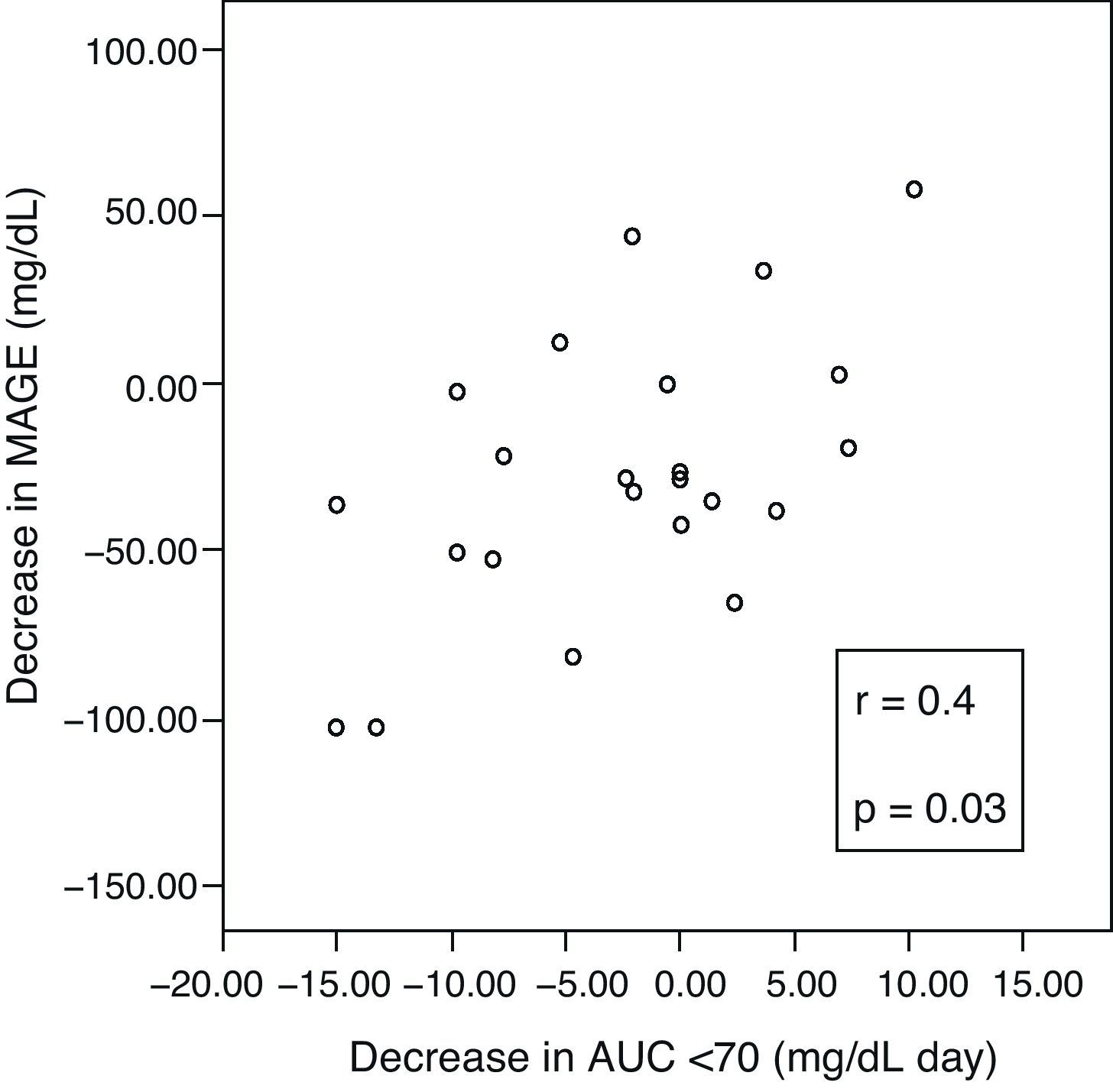
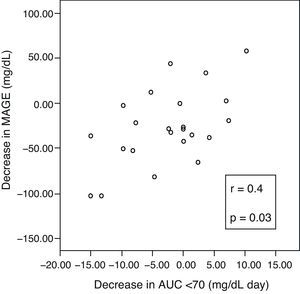
A positive correlation was found between incremental variations in ΔMAGE–ΔAUC (Fig. 3), which means that the greater the MAGE decrease, the greater the decrease in hypoglycemic time.
Quality of life (QOL)Overall quality of life and treatment satisfaction improved in all patients. A decrease in hypoglycemic excursions was perceived by 45.8% (11/24) of the patients.
DiscussionCSII therapy has traditionally been used in patients with T1DM with a strong dawn phenomenon, inadequate metabolic control, high glycemic variability, or severe hypoglycemia.33 However, since the advent of long-acting insulin analogs, there has been some controversy about its greater value in these situations. This is because, according to some authors, insulin analogs may achieve similar results at a lower cost. This concept has especially been questioned in hypoglycemia (one of its main indications),17 and two possible reasons have been suggested. One of them is that long-acting insulin analogs decrease the incidence of nocturnal hypoglycemia.34,35 The other reason is that several reports found no significant differences (between MIDs with insulin analogs and CSII) in the occurrence of hypoglycemia, as previously noted.20,21,24–26 However, most of these studies included a small sample size and were short in duration, which may have influenced the results.
The results of this and of other studies showed no significant differences (pre-CSII vs post-CSII) in hypoglycemic time after an overall sample of patients with T1DM was analyzed. We found, however, that CSII therapy may be extremely helpful in well selected cases (patients with more baseline AUC or MAGE). Similarly, Pickup and Sutton19 showed that the greatest decrease in the number of hypoglycemic episodes was seen in patients with a higher number of more severe hypoglycemic episodes before CSII. Giménez et al.27 also showed that treatment with CSII decreased hypoglycemic episodes in patients with recurrent severe to moderate hypoglycemic episodes. In agreement with these studies, we also found that patients with more hypoglycemic episodes at baseline benefit the most from CSII therapy because they achieve a greater episode reduction.
In addition, CSII therapy was shown to be a helpful tool for decreasing glycemic variability, as already reported in other studies.21,36 Moreover, patients with greater baseline variability showed better results in this study. This concept of glycemic variability has become very important in recent years, not only because of its potential and controversial relationship to the occurrence of microvascular complications, but also because of its relationship to the occurrence of hypoglycemia.37,38 In fact, it has been reported that, unlike as occurs with HbA1c, glycemic variability may explain up to 40–50% of variance in future hypoglycemic episodes.39 What this study contributes in this regard is that patients with greater variability are also those who achieve greater decreases in glycemic excursions. This is similar to what happens with HbA1c or hypoglycemia reduction, which is greater in patients with higher HbA1c values36 or more hypoglycemic episodes at baseline,18 respectively. This concept, not formulated to date, may allow us to add a new, clear indication for CSII, because glycemic variability is currently considered as an indication in some, but not all, guidelines.17
We also found a greater decrease in hypoglycemic episodes in patients with greater baseline variability. This supported the existence of a positive correlation between decreased glycemic variability and a reduction in hypoglycemic episodes. This finding has a great clinical relevance because it has been argued that patients with high glycemic variability often have sustained high HbA1c levels but are resistant to the intensification of insulin therapy because this could trigger the occurrence of hypoglycemia. Thus, if these “at risk” patients experience less hypoglycemic episodes on CSII, adequate HbA1c values will be easier to achieve.
Improvements in variability and hypoglycemic episodes were not only objectively documented, but were also subjectively perceived by the patients themselves, as shown by the QOL questionnaire.
This study therefore adds new, valuable information. First of all, this is because, unlike most studies, we did not focus on severe hypoglycemia, but on episodes not requiring third-party care. Such episodes represent a risk factor for severe hypoglycemia and are more frequent and difficult to prevent. Second, since the reference method for studying variability has not yet been elucidated,40,41 the data were analyzed with three different measures of glycemic variability (SD, M-value, and MAGE). This is in contrast to previously reported studies, which used one or two variables. On the other hand, we used CGM, which is considered the gold standard for identifying glycemic excursions. In fact, this is the first study to assess glycemic variability and hypoglycemic time using several specific parameters and CGM in all patients treated with CSII. In 2006, Pickup et al.36 showed CSII to decrease glycemic variability as estimated using SD from the self-monitoring of capillary blood glucose and, therefore, recommended the use of CGM. Two years later, in 2008, Simon et al.42 studied glycemic variability and hypoglycemia using CGM, but did not use specific variables (only the percentage of values >180mg/dL or <60mg/dL). Finally, Bruttomesso21 assessed glycemic variability and hypoglycemic episodes using three different parameters (MAGE, SD, ADRR), but CGM was used in less than one third of the patients (11/39) and for 34h only.
Despite these advantages, this study has various limitations. Like most other studies published on this subject, our study included a small sample, although it was adequate for reaching statistical significance using non-parametric methods. The glycemic variability parameters used correspond to overall glycemic variability measures, to which other known specific measures of intra-day glycemic variability such as continuous overall net glycemic action (CONGA) or inter-day glycemic variability, such as the mean of daily difference (MODD) or CONGA24h, could have been added. As regards the study of hypoglycemic episodes, there are several disadvantages. On the one hand, the results of capillary blood glucose tests performed by the patients themselves were not analyzed. In addition, our study was based on the AUC<70mg/dL from a single monitoring day. Moreover, early CGM devices, mainly CGMS® System Gold™, are less precise for detecting blood glucose values lower than 70mg/dL (although they are valid and widely used for this purpose27,38). Finally, no control group was available for a comparison of the results. The main reason for this was that our objective was not to compare CSII vs MIDs, but to assess whether CSII could be a useful treatment in patients with great variability. We are, however, aware that control for other variables related to the intervention (a group given the same diabetes education, attending the same visits, etc.) would have been convenient.
To sum up, CSII is an extremely helpful therapy for patients with T1DM, particularly those with great glycemic variability and/or frequent hypoglycemic episodes. The greatest decreases in glycemic variability and hypoglycemic episodes are achieved in such patients. Patients eligible for CSII should, therefore, be carefully selected so that they may obtain maximum benefits from insulin pump therapy.
Conflict of interestThe authors state that they have no conflicts of interest.
We would like to thank Sociedad Gallega de Endocrinología y Nutrición for the funding of this study.
Please cite this article as: Prieto-Tenreiro A, et al. Beneficios de la terapia con infusión subcutánea continua de insulina en pacientes diabéticos tipo 1 que presentan gran variabilidad glucémica. Endocrinol Nutr. 2012;59:246–53.