To assess the relationship between primary hypothyroidism and subclinical atherosclerosis and its potential changes with L-thyroxine replacement therapy.
MethodsA prospective cohort study including 101 patients with primary hypothyroidism and 101 euthyroid patients as controls was conducted from July 2011 to December 2013. Clinical, anthropometrical, biochemical, and ultrasonographic parameters were assessed at baseline and after one year of L-thyroxine replacement therapy.
ResultsAt baseline, hypothyroid patients had significantly greater values of blood pressure, total cholesterol, VLDL cholesterol, left ventricular mass, epicardial fat, and carotid intima-media thickness as compared to controls. Total cholesterol, VLDL cholesterol, ventricular diastolic function, epicardial fat, carotid intima-media thickness, carotid local pulse wave velocity, pressure strain elastic modulus, and β arterial stiffness index showed a significant and positive correlation with TSH levels. After one year of replacement therapy, patients with hypothyroidism showed changes in total cholesterol, VLDL cholesterol, TSH, carotid intima-media thickness, and arterial stiffness parameters.
ConclusionsPrimary hypothyroidism is characterized by an increased cardiovascular risk. In these patients, L-thyroxine replacement therapy for one year is related to decreased dyslipidemia and improvement in markers of subclinical carotid atherosclerosis.
Determinar la relación del hipotiroidismo primario con la aterosclerosis carotídea subclínica y sus posibles modificaciones con la terapia sustitutiva.
MétodosSe realizó un estudio de cohorte prospectivo que incluyó 101 pacientes con diagnóstico de hipotiroidismo primario y 101 pacientes eutiroideos como controles, desde julio del año 2011 hasta diciembre del 2013. Se incluyeron variables clínicas, antropométricas, bioquímicas y ultrasonográficas, evaluadas al inicio del estudio y al año de tratamiento sustitutivo con levotiroxina sódica.
ResultadosLos sujetos afectos de hipotiroidismo al inicio mostraron valores significativamente mayores de tensión arterial, colesterol total, colesterol VLDL, masa del ventrículo izquierdo, grasa epicárdica y grosor íntima-media carotídeo, respecto a los controles. El colesterol total, el colesterol VLDL, la función diastólica ventricular, la grasa epicárdica, el grosor íntima media, la velocidad de propagación del pulso carotídeo, la elastancia y el índice de rigidez arterial β mostraron una correlación positiva y significativa con las concentraciones de TSH. Al año de tratamiento, los pacientes afectos de hipotiroidismo tuvieron modificaciones para el colesterol total, el colesterol VLDL, la TSH, el grosor íntima-media y los parámetros de rigidez arterial.
ConclusionesLos pacientes afectos de hipotiroidismo primario se caracterizan por mayor riesgo cardiometabólico. La sustitución con levotiroxina sódica en estos pacientes se relaciona con una mejoría de la dislipidemia, y mejoría de los indicadores de aterosclerosis carotídea subclínica al año de iniciado el tratamiento.
Hypothyroidism results from deficient thyroid hormone secretion and is associated with comorbid conditions such as high blood pressure, dyslipidemia, and ischemic heart disease. Hypothyroidism is more prevalent between the third and sixth decades of life and in females. Its incidence in the general population ranges from 1% to 2%, and reaches 6–7% in females over 60 years of age. In Cuba, hypothyroidism is the second leading endocrine disease after diabetes mellitus.1
In patients with hypothyroidism increased total and LDL cholesterol levels and decreased HDL cholesterol levels have been found. It is thus clear that the condition involves an atherogenic profile, which may increase vascular risk in those who suffer from it.2
Cardiovascular risk appears to be independently associated with subclinical hypothyroidism in patients over 65 years of age. Women with hypothyroidism have a two-fold greater risk of developing atherosclerosis and a greater prior history of myocardial infarction as compared to controls. An increased incidence of peripheral artery disease, diastolic and systolic left ventricular dysfunction, and ischemic coronary events has also been reported in patients with subclinical hypothyroidism.3
It is currently known that cardiovascular changes in thyroid dysfunction are not restricted to clinically evident forms of dysfunction. There is considerable evidence to suggest that the cardiovascular system responds to the minimal but persistent changes in circulating thyroid hormone levels which are characteristic of subclinical thyroid dysfunction.4
Both clinical and subclinical hypothyroidism are related to a reversible state of endothelial dysfunction which may be responsible for high blood pressure and an increased risk of atherosclerosis. As TSH increases, flow-mediated vasodilation decreases, suggesting the presence of endothelial dysfunction. On the other hand, the cardiovascular change most commonly found in hypothyroidism is left ventricular (LV) diastolic dysfunction, characterized by slow myocardial relaxation and delayed ventricular filling.5
The increased risk of atherosclerosis in hypothyroidism mainly results from insulin resistance, and is linked to a low-grade chronic inflammation state promoted by the large quantity of adipokines secreted by adipose tissue, including tumor necrosis factor alpha and interleukin-6, which accelerate athrerosclerosis.4
Failure to adjust levothyroxine levels results in changes due to either excess or deficient drug dosages. However, progressive improvement of the clinical condition after replacement therapy has been shown by improved quality of life and symptom remission in patients.4
Although different observational studies have shown a relationship between dyslipidemia, atherosclerotic cardiovascular disease, and thyroid dysfunction, the results are still conflicting,6–8 particularly for subclinical disease. Moreover, few studies are available suggesting that early atherosclerosis detected in patients with subclinical thyroid hypofunction is reversible with levothyroxine treatment and the maintenance of a stable thyroid function,9–11 especially as regards potential changes in carotid artery stiffness.12,13
The aim of this study was therefore to identify the relationship between subclinical carotid atherosclerosis and primary hypothyroidism, as well as their potential changes with replacement therapy.
MethodsA prospective, observational, cohort study was conducted in which two comparison groups were consecutively enrolled: (1) 101 patients with documented diagnosis of primary hypothyroidism at baseline: idiopathic (n=47; 46.5%), due to chronic thyroiditis (n=33; 32.7%), postoperative (n=12; 11.9%), and subsequent to the administration of I131 (n=9; 8.9%); they were all treated with replacement doses of levothyroxine sodium (1.5–1.9μg/kg, starting at half the dose, which was subsequently increased until the dose required to maintain patients clinically euthyroid and with TSH levels ranging from 0.27 to 3.75mIU/L was reached); and (2) 101 euthyroid subjects as controls. Both groups consisted of subjects aged 25–40 years. The patients attended the endocrinology outpatient clinics of Hospital Universitario Dr. Miguel Enríquez and the National Endocrinology Institute from July 2011 to December 2013. There were no patients with hypothyroidism due to iodine excess or deficiency because there are no iodine-deficient regions in Cuba and drugs containing iodine, such as amiodarone, are not commonly used at these ages. Patients with thyroid development abnormalities, such as hypoplasia, aplasia, and abnormal locations with hypofunction were not enrolled because these are often discovered in childhood and treated with levothyroxine, so that replacement has already been achieved at these ages. Patients with a history of diabetes, ischemic heart disease, cerebrovascular disease, renal failure, or other chronic diseases that promoted the occurrence of the development of atherosclerosis were excluded. The study was conducted in accordance with the recommendations in the Declaration of Helsinki and was approved by the ethics committee of our institution.
The exclusion criteria during follow-up included: failure to attend follow-up visits, treatment discontinuation for longer than two weeks, the occurrence of significant adverse effects attributable to treatment, and the voluntary withdrawal of treatment.
Clinical (age, sex, smoking status), anthropometric (waist circumference [WC] and the body mass index [BMI]), biochemical (blood glucose, total cholesterol, VLDL cholesterol, HDL cholesterol, triglycerides, creatinine, uric acid, aspartate aminotransferase [AST], alanine aminotransferase [ALT], fasting plasma insulin, and TSH), and ultrasonographic (carotid intima-media thickness [CIMT], arterial stiffness parameters [carotid local pulse wave velocity {LPWV}, elastance {Ep}, arterial compliance {CA} and index β], left atrial [LA] volume, left ventricular [LV] mass index, E/e′ ratio, and epicardial fat) variables were collected.
Patients underwent the complete study program when they first attended the clinic. TSH levels were measured monthly, and the dosage was adjusted until an euthyroid state was achieved. TSH measurements were subsequently performed every three months, and the full study program was again performed one year after control had been achieved.
Collection of anthropometric parameters- -
Waist circumference: the abdominal perimeter was measured in patients with no clothes at the mid-point between the last costal arch and the anterosuperior iliac crest.
- -
The body mass index: this was calculated using the Quetelet formula: weight in kg/(height in m)2.
In all participants, blood (10mL) was drawn by puncture in the cubical vein into tubes with EDTA, heparin, and dry gel for serum. Blood glucose, total cholesterol, and triglycerides were measured using as reagents RapiGluco-Test, Colestest, and Monotriglitest, respectively, produced by the company Carlos J. Finlay (Havana, Cuba). Blood glucose was measured in Eppendorf equipment using a colorimetric enzymatic method, and cholesterol, triglycerides, AST, ALT, and uric acid in Hitachi 7170 A equipment (Tokyo, Japan). HDL and VLDL cholesterol were measured using C-HDL InmunoFS and VLDL-C Select FS as reagents, respectively by quantitative in vitro measurement in serum or plasma in photometric equipment (immunoturbidimetric test) from DiaSys Diagnostic Systems GmbH (Holzheim, Germany).
Insulin was measured using a radioimmunoassay (competitive solid-phase Coat-A-CountInsulin [DPC] radioimmunoassay) with a sensitivity of 5μIU/mL, a normal range of 5–35μIU/mL, and inter- and intra-assay coefficients of variation of 6.2% and 7.1%, respectively.
Ultrasensitive TSH was measured using a competitive electrochemiluminescence immunoassay (Modular Analytics E 170, Roche). The lowest limit of detection was 0.005μIU/mL, and coefficients of variation were 7.2% for a concentration of 0.035μIU/mL and 3.3% for a concentration of 3.66μIU/mL during the study period. In the radioimmunoassay, the coefficient of variation of the quality control usually ranged from 6% to 9%, the typical range for this type of immunoradiological method.
Collection of ultrasonographic parametersUltrasound examination was performed using Aloka Alfa-10 equipment (MitakaShi, Tokyo, Japan) with an S5-1 transducer fitted with harmonic imaging with a frequency of 1.3–3.6MHz. The examination was performed by a professional with level III experience of the American Society of Echocardiography who was blinded to patient data at all times.
- -
Epicardial fat: Epicardial fat was measured bi-dimensionally in the parasternal long-axis views, taking as the reference for sections the aortic valve plane and the parasternal short axis at the papillary muscle level. To determine epicardial fat anterior to RV, the values obtained from the parasternal long- and short-axis views were averaged. Epicardial fat was measured in the end-diastolic period of the cardiac cycle. Epicardial fat was defined as the echolucent space (with echorefringent elements inside suggesting fat) between the echodense line of the visceral pericardium and the epicardium of the right ventricular wall.
- -
LV mass index: This was calculated from measurements of LV epicardial and endocardial areas in the parasternal short-axis view at midventricular level. The LV area was subsequently measured using the truncated ellipsoid method described by Schiller et al.14 The equipment automatically reported LV mass in grams.
- -
E/e′ ratio: this is the ratio between LV diastolic filling velocity as determined by mitral flow and mitral annular displacement velocity by tissue Doppler. This ratio assesses diastolic LV function and is used to calculate filling pressures.
- -
Arterial stiffness parameters: These were determined using e-Tracking software built into the ultrasound equipment Aloka Alfa-10 (MitakaShi, Tokyo, Japan). Using the same procedure as for measuring CIMT, each carotid artery was separately examined, the name of the vessel being selected in the equipment menu, enabling the echo-tracking function in the control panel, and disabling the harmonic function because of the proximity of vascular structures to the transducer. For the analysis, systolic (SBP) and diastolic blood pressure (DBP) were measured using a mercury sphygmomanometer upon patient arrival, and these values were entered in the software menu.
The echo-tracking cursor was positioned in the center of the longitudinal image of the artery. This system detects movement of the vessel caused by wave displacement through two cursors, of which one is positioned in the anterior wall and the other in the posterior wall of the artery. The maximum and minimum vessel diameters are obtained for 10 cardiac cycles. Once primary information is stored in the equipment, the software automatically determines the values of local pulse wave velocity (LPWV) in the carotid artery, the pressure exerted by blood to deform the vessel (Ep), the volume change by blood pressure (AC), and the arterial stiffness index β (the change in arterial diameter in systole as compared to diastole during the cardiac cycle). Three measurements were made, and the average value was taken.
Carotid intima-media thickness: Patients were placed in a supine position with the neck slightly turned to one side, and a linear transducer with a frequency of 7.5MHz was located longitudinally with respect to the common carotid artery (the latero-superior position of the neck inside the sternocleidomastoid muscle), with the electrocardiogram signal enabled to obtain vascular images at the end of diastole, with visualization at a depth of 4cm. To measure CIMT, both common carotid arteries were examined in a 10mm segment distal to the point of the emergence of the carotid bulb. Measurements were made at posterior wall level using semi-automatic margin detection software.
Statistical analysisQualitative variables were expressed as absolute numbers and percentages, and quantitative variables as mean and standard deviation. A Chi-square test and a Student's t test were used to test differences in qualitative and quantitative variables, respectively. A correlation of the biochemical, echocardiographic, and ultrasonographic variables with TSH was determined using Pearson's correlation coefficient. A multifactorial ANOVA test was used to assess differences in the evaluated parameters in patients with hypothyroidism before and after replacement therapy. A value of p<0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 20.0 software (SPSS Inc., Chicago, USA).
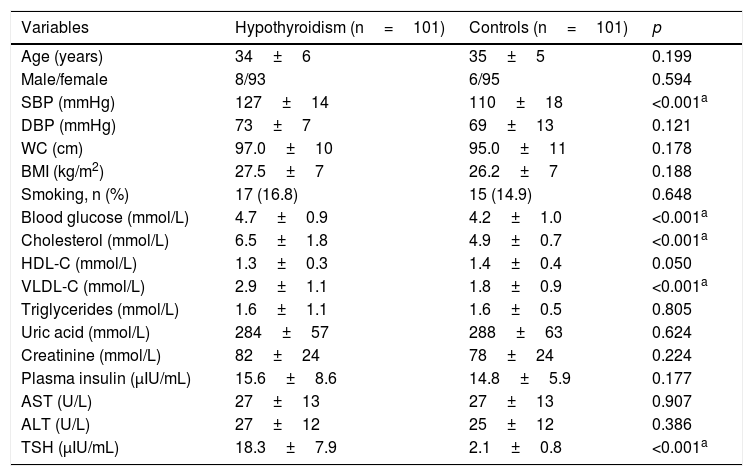
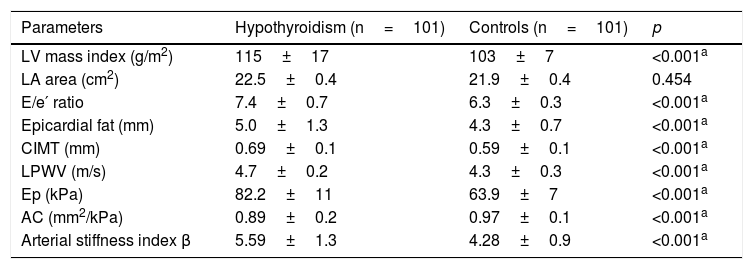
ResultsThe study sample consisted of 202 patients divided into two groups (cases [n=101] versus controls [n=101]) with a similar mean age (32±5 versus 33±5 years, respectively) and no sex differences (91.2% versus 94.1% females, respectively). Patients diagnosed with hypothyroidism had significantly higher values of SBP (127±14 versus 110±18mmHg), fasting blood glucose (4.7±0.9 versus 4.2±1.0mmol/L), total cholesterol (7.5±1.8 versus 4.9±0.7mmol/L), and VLDL cholesterol (2.9±1.1 versus 1.8±0.9mmol/L) (Table 1). On the other hand, except for the LA area, the LV mass index, the E/e′ ratio, epicardial fat thickness, CIMT, and carotid artery stiffness parameters were significantly higher in subjects with hypothyroidism as compared to controls (Table 2).
Clinical, anthropometric, and biochemical variables in patients with hypothyroidism as compared to controls.
| Variables | Hypothyroidism (n=101) | Controls (n=101) | p |
|---|---|---|---|
| Age (years) | 34±6 | 35±5 | 0.199 |
| Male/female | 8/93 | 6/95 | 0.594 |
| SBP (mmHg) | 127±14 | 110±18 | <0.001a |
| DBP (mmHg) | 73±7 | 69±13 | 0.121 |
| WC (cm) | 97.0±10 | 95.0±11 | 0.178 |
| BMI (kg/m2) | 27.5±7 | 26.2±7 | 0.188 |
| Smoking, n (%) | 17 (16.8) | 15 (14.9) | 0.648 |
| Blood glucose (mmol/L) | 4.7±0.9 | 4.2±1.0 | <0.001a |
| Cholesterol (mmol/L) | 6.5±1.8 | 4.9±0.7 | <0.001a |
| HDL-C (mmol/L) | 1.3±0.3 | 1.4±0.4 | 0.050 |
| VLDL-C (mmol/L) | 2.9±1.1 | 1.8±0.9 | <0.001a |
| Triglycerides (mmol/L) | 1.6±1.1 | 1.6±0.5 | 0.805 |
| Uric acid (mmol/L) | 284±57 | 288±63 | 0.624 |
| Creatinine (mmol/L) | 82±24 | 78±24 | 0.224 |
| Plasma insulin (μIU/mL) | 15.6±8.6 | 14.8±5.9 | 0.177 |
| AST (U/L) | 27±13 | 27±13 | 0.907 |
| ALT (U/L) | 27±12 | 25±12 | 0.386 |
| TSH (μIU/mL) | 18.3±7.9 | 2.1±0.8 | <0.001a |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; WC, waist circumference; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Ultrasonographic and echocardiographic parameters in patients with hypothyroidism and controls.
| Parameters | Hypothyroidism (n=101) | Controls (n=101) | p |
|---|---|---|---|
| LV mass index (g/m2) | 115±17 | 103±7 | <0.001a |
| LA area (cm2) | 22.5±0.4 | 21.9±0.4 | 0.454 |
| E/e′ ratio | 7.4±0.7 | 6.3±0.3 | <0.001a |
| Epicardial fat (mm) | 5.0±1.3 | 4.3±0.7 | <0.001a |
| CIMT (mm) | 0.69±0.1 | 0.59±0.1 | <0.001a |
| LPWV (m/s) | 4.7±0.2 | 4.3±0.3 | <0.001a |
| Ep (kPa) | 82.2±11 | 63.9±7 | <0.001a |
| AC (mm2/kPa) | 0.89±0.2 | 0.97±0.1 | <0.001a |
| Arterial stiffness index β | 5.59±1.3 | 4.28±0.9 | <0.001a |
LA, left atrium; AC, arterial compliance; Ep, elastance; CIMT, carotid intima-media thickness; LV, left ventricle; LPWV, local (carotid) pulse wave velocity.
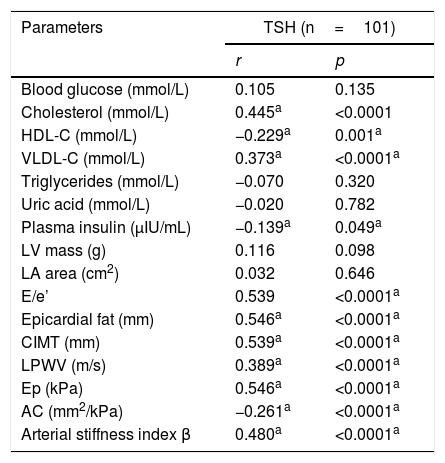
Table 3 shows the correlation between TSH and the study variables. Total cholesterol, VLDL cholesterol, the E/e′ ratio, epicardial fat, CIMT, LPWV, elastance, and the arterial stiffness index β showed a positive significant correlation with TSH levels, while HDL cholesterol and arterial compliance showed a negative significant correlation.
Correlation between TSH and biochemical and ultrasonographic parameters in patients with hypothyroidism.
| Parameters | TSH (n=101) | |
|---|---|---|
| r | p | |
| Blood glucose (mmol/L) | 0.105 | 0.135 |
| Cholesterol (mmol/L) | 0.445a | <0.0001 |
| HDL-C (mmol/L) | −0.229a | 0.001a |
| VLDL-C (mmol/L) | 0.373a | <0.0001a |
| Triglycerides (mmol/L) | −0.070 | 0.320 |
| Uric acid (mmol/L) | −0.020 | 0.782 |
| Plasma insulin (μIU/mL) | −0.139a | 0.049a |
| LV mass (g) | 0.116 | 0.098 |
| LA area (cm2) | 0.032 | 0.646 |
| E/e’ | 0.539 | <0.0001a |
| Epicardial fat (mm) | 0.546a | <0.0001a |
| CIMT (mm) | 0.539a | <0.0001a |
| LPWV (m/s) | 0.389a | <0.0001a |
| Ep (kPa) | 0.546a | <0.0001a |
| AC (mm2/kPa) | −0.261a | <0.0001a |
| Arterial stiffness index β | 0.480a | <0.0001a |
LA, left atrium; AC, arterial compliance; Ep, elastance; CIMT, carotid intima-media thickness; LV, left ventricle; LPWV, local (carotid) pulse wave velocity.
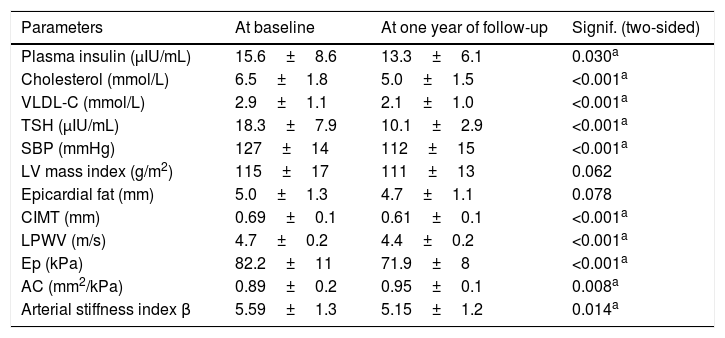
Table 4 shows differences in biochemical, ultrasonographic, and echocardiographic parameters in patients with hypothyroidism at baseline and after one year of treatment. Significant decreases were found in SBP, total cholesterol, VLDL cholesterol, TSH, CIMT, LPWV, AC, Ep, and the stiffness index β after one year of treatment as compared to baseline.
Clinical, biochemical, and ultrasonographic parameters of interest in patients with hypothyroidism at baseline and after one year of follow-up.
| Parameters | At baseline | At one year of follow-up | Signif. (two-sided) |
|---|---|---|---|
| Plasma insulin (μIU/mL) | 15.6±8.6 | 13.3±6.1 | 0.030a |
| Cholesterol (mmol/L) | 6.5±1.8 | 5.0±1.5 | <0.001a |
| VLDL-C (mmol/L) | 2.9±1.1 | 2.1±1.0 | <0.001a |
| TSH (μIU/mL) | 18.3±7.9 | 10.1±2.9 | <0.001a |
| SBP (mmHg) | 127±14 | 112±15 | <0.001a |
| LV mass index (g/m2) | 115±17 | 111±13 | 0.062 |
| Epicardial fat (mm) | 5.0±1.3 | 4.7±1.1 | 0.078 |
| CIMT (mm) | 0.69±0.1 | 0.61±0.1 | <0.001a |
| LPWV (m/s) | 4.7±0.2 | 4.4±0.2 | <0.001a |
| Ep (kPa) | 82.2±11 | 71.9±8 | <0.001a |
| AC (mm2/kPa) | 0.89±0.2 | 0.95±0.1 | 0.008a |
| Arterial stiffness index β | 5.59±1.3 | 5.15±1.2 | 0.014a |
AC, arterial compliance; Ep, elastance; CIMT, carotid intima-media thickness; DBP, diastolic blood pressure; SBP, systolic blood pressure; LPWV, local (carotid) pulse wave velocity.
The most significant and novel findings in our study were that patients with hypothyroidism have greater cardiometabolic risk as compared to controls, and that, in young hypothyroid patients, replacement therapy for one year decreases lipid changes and subclinical carotid atherosclerosis.
Lipid metabolism is impaired in hypothyroidism, and approximately 90% of patients with overt hypothyroidism have elevated levels of total and VLDL cholesterol.3 Similar results were found in this study, which showed significantly higher total and VLDL cholesterol levels in patients with hypothyroidism as compared to controls. Subclinical hypothyroidism has also been associated with increased VLDL and total cholesterol levels in several cross-sectional studies,4,11,15 while Nakova et al. reported significant increases in mean triglyceride levels and the total cholesterol/HDL cholesterol ratio in 69 patients with subclinical hypothyroidism.16
Similarly, TSH levels were found to have a proportional and significant relationship with insulin, total cholesterol, and VLDL cholesterol levels, and an inverse relationship with HDL cholesterol. These results show a potential relationship between hypothyroidism and metabolic syndrome. On the other hand, in agreement with our study, Adrees et al. showed significant decreases in total, LDL, and VLDL cholesterol levels with replacement therapy in patients with subclinical hypothyroidism.10
As regards the echocardiographic and ultrasonographic parameters, our study found a significant relationship between primary hypothyroidism and the E/e′ ratio, epicardial fat, CIMT, Ep, the stiffness index β, and an inversely proportional relationship to AC. These findings relate subclinical primary hypothyroidism and TSH levels to LV diastolic dysfunction, carotid artery stiffness, and epicardial fat, an emerging marker of cardiometabolic risk17 which has been related in prior studies in Hispanic populations with metabolic syndrome18,19 and carotid20 and coronary atherosclerosis.21 In this regard, in the only study found in the literature on this subject, Korkmaz et al.22 found a significantly greater epicardial fat thickness in hypothyroid patients as compared to controls (3.6±0.9 versus 2.8±1.4mm; p=0.005). To our knowledge, this is the first study to assess the potential changes in epicardial fat with replacement therapy in patients with hypothyroidism, although a non-significant reduction in epicardial fat was only seen after one year of treatment.
Various authors have also reported a relationship between subclinical hypothyroidism and CIMT. A recent meta-analysis including eight studies with a total of 3602 patients23 showed significantly higher CIMT values in patients with subclinical hypothyroidism as compared to a euthyroid group. Adrees et al. reported findings similar to those of our study with regard to CIMT reduction with replacement therapy in 56 women in whom CIMT decreased by 13% as compared to baseline values after 18 months of replacement therapy.10 Kim et al. similarly reported a significant decrease in CIMT (from 0.67±0.11 to 0.60±0.10mm) after hormone replacement therapy for 18 months in 28 patients with hypothyroidism.11 By contrast, Dias et al. found no significant CIMT changes in 32 women with subclinical hypothyroidism during one year of treatment.24
Left ventricular diastolic dysfunction at rest and both systolic and diastolic dysfunction with exercise also occur in hypothyroidism. Impaired left ventricular diastolic function due to slower myocardial relaxation and impaired early ventricular filling has been shown. T4 replacement therapy resolves these functional abnormalities and improves both systolic and diastolic function. Changes in LV diastolic dysfunction at rest have also been shown in patients with subclinical hypothyroidism, with improved response to T4 replacement therapy.4
This diastolic function involvement may also be related to the characteristic increase in arterial stiffness in these patients. The close relationship between hypothyroidism and arterial stiffness, evaluated with different measurement methods (radial artery tonometry,12 oscillometric method in brachial artery,13 and carotid ultrasound25), has been shown in several studies. In this regard, Tian et al. showed in 93 patients significantly higher values of the carotid arterial stiffness index β in patients with hypothyroidism as compared to controls,25 and a positive correlation between this arterial stiffness parameter and TSH levels.
Koren et al.12 showed in 30 patients with subclinical hypothyroidism a significant decrease in the augmentation index, an additional parameter indicating arterial stiffness, by 3.0% as compared to baseline values after seven months of replacement therapy.
Another interesting finding in our study was the higher SBP values in patients with hypothyroidism, which showed significant changes with replacement therapy. The Gao et al. meta-analysis23 also found a relationship between subclinical hypothyroidism and increased SBP. It has been noted, however, that up to 20% of patients with overt hypothyroidism show significant diastolic hypertension related to increased arterial stiffness, and this has been reported to improve with T4 replacement therapy.3,4
There were no external factors influencing the changes seen at one year of treatment, that is, patients with hypothyroidism were only treated with levothyroxine at replacement doses.
In conclusion, patients with primary hypothyroidism are characterized by greater cardiometabolic risk, increased epicardial fat, and subclinical carotid atherosclerosis as compared to euthyroid patients. In these patients, replacement therapy with levothyroxine sodium is related to improvements in dyslipidemia and carotid atherosclerosis markers after one year of treatment.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: del Busto-Mesa A, Cabrera-Rego JO, Carrero-Fernández L, Hernández-Roca CV, González-Valdés JL, de la Rosa-Pazos JE. Modificaciones de la rigidez arterial, el grosor íntima-media carotídeo y la grasa epicárdica con tratamiento sustitutivo en el hipotiroidismo. Endocrinol Nutr. 2015;62:270-276.