The primary study objective was to assess the proportion of patients with type 2 diabetes and an HbA1c value ≤6.5% from the start of insulin therapy to five years later in the outpatient setting in Spain.
Materials and methodsThis was an observational, multicenter, naturalistic study with retrospective collection of clinical data. Investigators were endocrinologists or internal medicine specialists from all over Spain. During standard clinical care, patients started insulin therapy, which was continued for at least 5 years.
ResultsThe clinical records of 405 patients were reviewed. The final analysis set included records from 346 patients. At baseline (start of insulin therapy), 51.2% of patients were female; mean (SD) age was 64.6 (9.0) years; body mass index, 29.8 (4–5)kg/m2; time since diagnosis, 8.8 (6.8) years; HbA1c, 9.4% (1.5); fasting glucose, 223.7 (55.9)mg/dL; and mean 2-hour postprandial glucose, 293.6 (71.0)mg/dL. When insulin therapy was started, <1.0% of patients had an HbA1c value ≤6.5%. At 5 years, 10.3% of patients achieved the HbA1c goal of ≤6.5% (mean, 7.72%). All glucose parameters (HbA1c, fasting glucose, and 2-hour postprandial glucose) improved at 5 years as compared to values at the start of insulin therapy.
ConclusionsGlucose parameters improved over time in patients with type 2 diabetes in this naturalistic study. However, blood glucose control exceeded the internationally recommended target values. These results therefore suggest that there is still some margin for improvement in outpatient care in Spain.
El objetivo principal fue valorar el porcentaje de pacientes con diabetes tipo 2 y un nivel de HbA1c ≤6,5% desde el inicio de la insulinoterapia y hasta 5 años después en el marco ambulatorio en España.
Materiales y métodosSe trató de un estudio multicéntrico, observacional y naturalista en el que los datos clínicos se recopilaron de forma retrospectiva. Los investigadores participantes en este estudio eran endocrinólogos o médicos internistas de toda España. En el transcurso de la atención médica habitual los pacientes iniciaron un tratamiento con insulina que continuó durante un periodo mínimo de 5 años.
ResultadosSe llevó a cabo una revisión de las historias clínicas de 405 pacientes. En la muestra final para el análisis se incluyeron las historias clínicas de 346 pacientes. Al inicio del estudio (comienzo de la insulinoterapia) el 51,2% de los pacientes eran mujeres, con una media (DE) de edad de 64,6 (9,0) años; un índice de masa corporal de 29,8 (4,5)kg/m2; un tiempo transcurrido desde el diagnóstico de 8,8 (6,8) años; un nivel de HbA1c del 9,4% (1,5); un nivel de glucemia en ayunas de 223,7 (55,9)mg/dl y una media de glucemia a las 2h postingesta de 293,6 (71,0)mg/dl. Al inicio de la insulinización <1% de los pacientes tenían una HbA1c ≤6,5%. A los 5 años el 10,3% de los pacientes alcanzó el objetivo de HbA1c ≤6,5% (media: 7,72%). Todos los parámetros glucémicos revisados (HbA1c, glucemia en ayunas y glucemia a las 2h postingesta) mejoraron a los 5 años en comparación con los mismos valores al inicio de la insulinoterapia.
ConclusionesEn este estudio naturalista los parámetros glucémicos mejoraron a lo largo del tiempo en este grupo de pacientes con diabetes tipo 2. Sin embargo, el control glucémico excedió los valores objetivo recomendados por las sociedades científicas. Por tanto, estos resultados indican que existe todavía un margen de mejora en la atención ambulatoria de estos pacientes en España.
Because of the progressive nature of type 2 diabetes mellitus, early and effective treatment is critical to prevent or delay complications of diabetes. Organizations such as the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) have proposed guidelines for medical care of patients with type 2 diabetes intended to promote such treatment which have been accepted by the international community. Such guidelines include early administration of treatment, parameters of blood glucose goals, and frequent monitoring.1,2 Under real life conditions, however, most patients with type 2 diabetes do not achieve the recommended blood glucose parameters,3 and there is a need for greater overall adherence to the established guidelines.4
Coinciding with the current pandemic of diabetes, the population of patients with type 2 diabetes is increasing in Spain.5–7 Prior observational studies showed that blood glucose control was suboptimal in most Spanish patients, with a limited proportion achieving the recommended blood glucose goals both in the group not receiving insulin4 and in the overall patients.8 Tests also suggest increased comorbidity associated to increased hyperglycemia in Spanish patients.9 In order to approach these concerns, it is essential to recognize the current therapeutic models and to identify the barriers preventing better patient care, and to continue to assess the overall resources available to guarantee sustainable results.
The observational, prospective study INSTIGATE was conduced to ascertain treatment practices under real life conditions.10,11 This study was designed to assess direct costs, use of healthcare resources, and metabolic control associated to insulin therapy in patients with type 2 diabetes from five European countries. Patients who started insulin therapy as part of their usual care were assessed, and medical care was the standard care given in the respective countries. During observation, mean blood glucose parameters exceeded the internationally recommended levels, including those found in the Spanish sample, thus contributing to risks related to diabetes.
Among the five countries participating in the INSTIGATE study, the Spanish sample showed high and sustained mean glucose levels from the start of the research and throughout the observation period.10,11 In order to assess diabetes care and to better characterize the clinical practice related to insulin therapy in a wide population of Spanish patients, the observational study EDIN was undertaken. This study assessed blood glucose control in patients with type 2 diabetes on outpatient treatment since the start of insulin therapy. The primary objective of this study was to assess the proportion of patients with type 2 diabetes with HbA1c ≤6.5% from the start of insulin therapy to five years later in the outpatient care setting in Spain. Thus, unlike the INSTIGATE study, the EDIN study was more focused on adherence to the internationally accepted blood glucose objectives in a large population of Spanish patients. Similarly to the INSTIGATE study, this study also reported patient characteristics and use of resources associated to insulin therapy.
Patients and methodsStudy designThis was a multicenter, observational, naturalistic study where clinical data were retrospectively collected. Investigators participating in the study were endocrinologists or internal medicine specialists from all over Spain usually caring for patients with diabetes in their outpatient clinics. Investigators collected data from patients who were receiving insulin therapy and with data available for at least five years since the start of such therapy. The study was completed with a cross-sectional visit of the patient.
The study was conducted in compliance with the ethical principles that have their origin in the Declaration of Helsinki. Local requirements related to ethical review of the study and regulatory study notifications were also met. All patients gave their written informed consent in accordance to local regulations, including the Spanish Act on Personal Data Protection (LOPD).
PatientsInclusion criteria were type 2 diabetes according to the ADA definition,12 at least 30 years of age, start of outpatient insulin therapy between 2000 and 2001, and at least five years of treatment. Exclusion criteria included type 1 diabetes, pregnancy, or gestational diabetes.
AssessmentsThe following clinical measurements were recorded at the start of insulin therapy and every year thereafter: anthropometric measurements (weight, height, and waist circumference), blood glucose control (HbA1c and fasting glucose), insulin therapy, cardiovascular risk factors (smoking, overweight or obesity, diagnosed hypertension and dyslipidemia), concurrent diseases, microvascular complications (diabetic retinopathy, nephropathy, or neuropathy), and macrovascular complications (coronary, cerebral, or peripheral artery disease). Change in insulin therapy was defined as one or more of the following: addition of a hypoglycemic drug, increase or decrease in the number of daily insulin injections, discontinuation of any type of insulin, or replacement of human insulin by an insulin analog or vice versa.
Information on use of healthcare resources related to type 2 diabetes was collected. Healthcare resources included use of test strips and home blood glucose monitoring, visits to day care unit, primary care visits, visits/referrals to specialists (endocrinologist, diabetologist, ophthalmologist, dietitian, podiatrist, or others), emergency room visits, hospital admissions and related care received (chemical tests, ophthalmoscopy, radiodiagnostic tests, or others).
Adverse events were not collected because of the study nature and design.
Statistical analysesThe primary objective of this study was to estimate the proportion of patients with type 2 diabetes who were in the blood glucose control goal (HbA1c ≤6.5%)13 on every year of insulin therapy up to the cross-sectional visit after approximately five years in the outpatient setting in Spain. A sample size of 675 patients was estimated for this study. Assuming 80% evaluable patients, the resulting 540 patients would provide a 95% confidence interval for determining the proportion of patients with an HbA1c level ≤6.5% with a maximum range width of 4.1%.
Statistical analyses were performed using version 8.2 of Statistical Analysis Software (SAS™ Institute, North Carolina, USA). Analyses reported in this study are mainly descriptive in nature. Continuous variables are given as mean, standard deviation (SD), median, maximum, and minimum. Categorical variables are given as absolute and relative frequencies and 95% confidence intervals. Time to the first change in insulin therapy was reported and calculated using the Kaplan–Meier method.
As this was a retrospective study and HbA1c was not always measured at each annual time point, a window of ±8 weeks was established for each annual assessment. If patients did not undergo the assessment within that time window, the value of the assessment for that year was considered missing. No special management was planned for missing data. Patients with missing values were included in the denominator to calculate percentages.
ResultsPatient history and characteristics at the start of insulin therapyThe clinical histories of 405 patients were reviewed. Histories from 28 patients were excluded due to non-compliance with inclusion criteria (n=16), absence of the investigator's signature in the case report form (n=11) and informed consent (n=1), and 377 validated histories were therefore obtained. Thirty-one of these histories were excluded because they did not include the HbA1c value at the start of insulin therapy. Thus, the final population for analysis included 346 clinical histories.
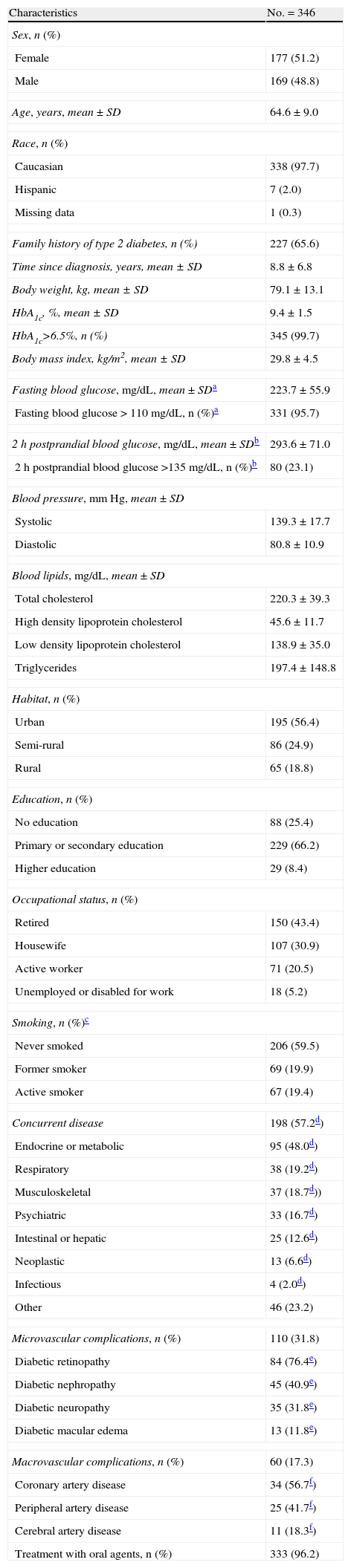
At the start of insulin therapy, 97.7% of the patients included in the analysis were Caucasians, 48.8% males and 51.2% females (Table 1). Fifty-six percent of the patients lived in urban areas, 66.2% had primary or secondary education, 43.4% were retired, and 59.5% had never smoked. Concurrent diseases were reported in 57.2% of the patients. Metabolic or endocrine diseases, such as lipid changes or thyroid diseases, were most common (48.0% of patients with concurrent diseases). Microvascular and macrovascular complications were found in 31.8% and 17.3% of the patients respectively. Microvascular complications consisted of diabetic retinopathy, nephropathy, neuropathy, and macular edema. The main macrovascular complication was coronary artery disease. Mean (SD) BMI (29.8 [4.5]kg/m2) and mean total cholesterol level (220.3 [39.3]mg/dL) revealed a population with overweight and dyslipidemia.
Demographic data and characteristics of patients at the start of insulin therapy.
| Characteristics | No.=346 |
| Sex, n (%) | |
| Female | 177 (51.2) |
| Male | 169 (48.8) |
| Age, years, mean±SD | 64.6±9.0 |
| Race, n (%) | |
| Caucasian | 338 (97.7) |
| Hispanic | 7 (2.0) |
| Missing data | 1 (0.3) |
| Family history of type 2 diabetes, n (%) | 227 (65.6) |
| Time since diagnosis, years, mean±SD | 8.8±6.8 |
| Body weight, kg, mean±SD | 79.1±13.1 |
| HbA1c, %, mean±SD | 9.4±1.5 |
| HbA1c>6.5%, n (%) | 345 (99.7) |
| Body mass index, kg/m2, mean±SD | 29.8±4.5 |
| Fasting blood glucose, mg/dL, mean±SDa | 223.7±55.9 |
| Fasting blood glucose>110mg/dL, n (%)a | 331 (95.7) |
| 2h postprandial blood glucose, mg/dL, mean±SDb | 293.6±71.0 |
| 2h postprandial blood glucose >135mg/dL, n (%)b | 80 (23.1) |
| Blood pressure, mmHg, mean±SD | |
| Systolic | 139.3±17.7 |
| Diastolic | 80.8±10.9 |
| Blood lipids, mg/dL, mean±SD | |
| Total cholesterol | 220.3±39.3 |
| High density lipoprotein cholesterol | 45.6±11.7 |
| Low density lipoprotein cholesterol | 138.9±35.0 |
| Triglycerides | 197.4±148.8 |
| Habitat, n (%) | |
| Urban | 195 (56.4) |
| Semi-rural | 86 (24.9) |
| Rural | 65 (18.8) |
| Education, n (%) | |
| No education | 88 (25.4) |
| Primary or secondary education | 229 (66.2) |
| Higher education | 29 (8.4) |
| Occupational status, n (%) | |
| Retired | 150 (43.4) |
| Housewife | 107 (30.9) |
| Active worker | 71 (20.5) |
| Unemployed or disabled for work | 18 (5.2) |
| Smoking, n (%)c | |
| Never smoked | 206 (59.5) |
| Former smoker | 69 (19.9) |
| Active smoker | 67 (19.4) |
| Concurrent disease | 198 (57.2d) |
| Endocrine or metabolic | 95 (48.0d) |
| Respiratory | 38 (19.2d) |
| Musculoskeletal | 37 (18.7d)) |
| Psychiatric | 33 (16.7d) |
| Intestinal or hepatic | 25 (12.6d) |
| Neoplastic | 13 (6.6d) |
| Infectious | 4 (2.0d) |
| Other | 46 (23.2) |
| Microvascular complications, n (%) | 110 (31.8) |
| Diabetic retinopathy | 84 (76.4e) |
| Diabetic nephropathy | 45 (40.9e) |
| Diabetic neuropathy | 35 (31.8e) |
| Diabetic macular edema | 13 (11.8e) |
| Macrovascular complications, n (%) | 60 (17.3) |
| Coronary artery disease | 34 (56.7f) |
| Peripheral artery disease | 25 (41.7f) |
| Cerebral artery disease | 11 (18.3f) |
| Treatment with oral agents, n (%) | 333 (96.2) |
SD, standard deviation.
Two-third of the patients reported a family history of type 2 diabetes. Patients had a suboptimal blood glucose control (mean [SD] HbA1c and fasting and 2h postprandial blood glucose: 9.4% [1.5%], 223.7 [55.9]mg/dL, 293.6 [71.0]mg/dL respectively) and did not meet the international recommendations for blood glucose control at the start of insulin therapy (100% of patients with HbA1c >6.5%, 95.7% with fasting blood glucose >110mg/dL, and 23.1% with 2h postprandial blood glucose >135mg/dL). At the start of insulin therapy, 96.2% of the patients were receiving hypoglycemic drugs.
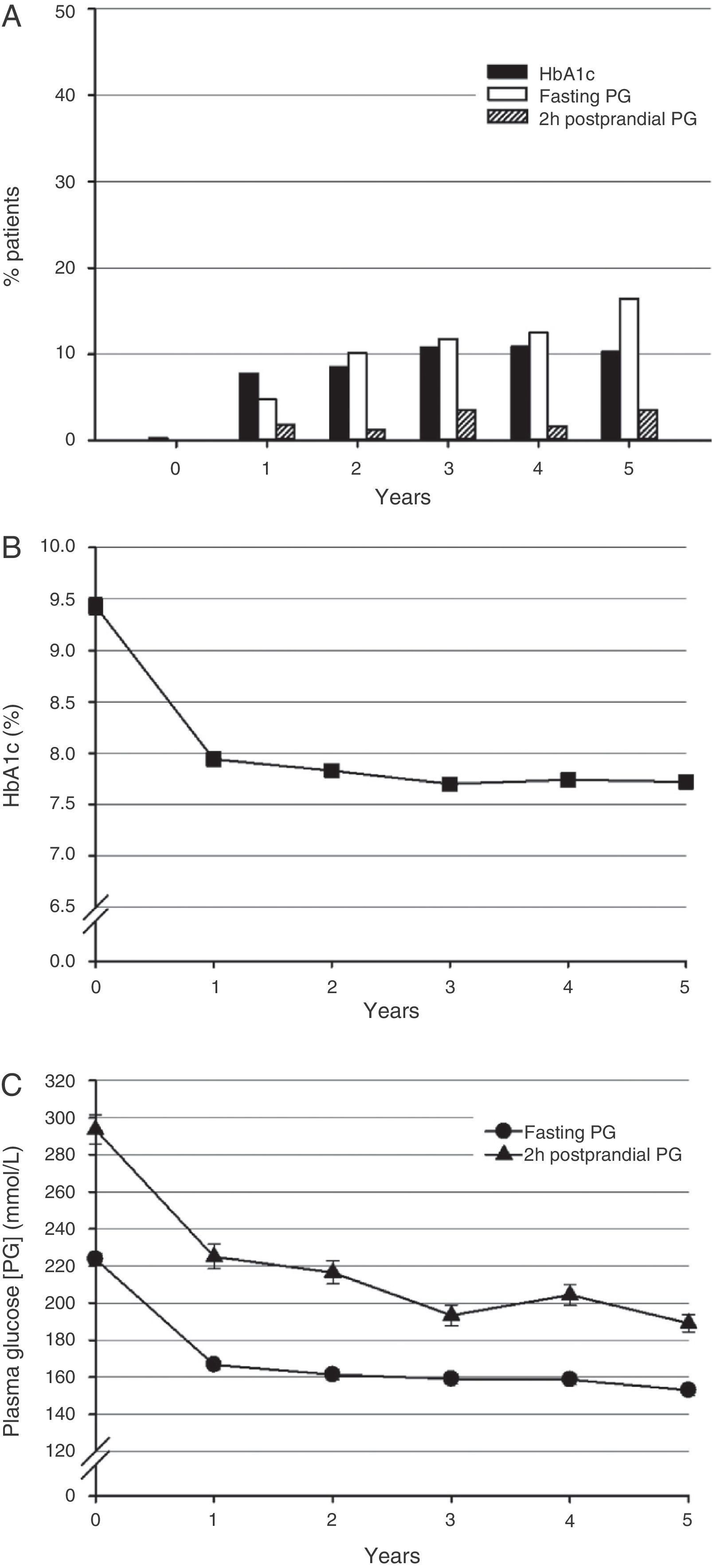
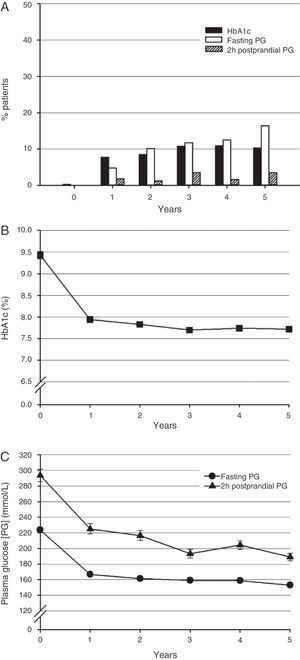
Glycosylated hemoglobinAfter five years, 10.3% of the patients (95% confidence interval [CI], 7.2 to 14.3; n=32) achieved an HbA1c level ≤6.5% (Fig. 1A). The proportions of patients who achieved the therapeutic goal after one, two, three, and four years were 7.8% (5.2–11.2), 8.5% (5.7–12.2), 10.8% (7.6–14.8), and 10.9% (7.6–14.9) respectively.
Blood glucose control during five years of insulin therapy. (A) Proportion of patients achieving the blood glucose control (HbA1c ≤6.5%) (black bars), fasting blood glucose (≤110mg/dl) (white bars), and 2h postprandial blood glucose (≤135mg/dL) (striped bars) goals (n=346 and 310 at the start of insulin therapy and at five years for each goal). (B) HbA1c levels (n=346 and 307 at the start of insulin therapy and at five years). (C) Fasting (circles) (n=331 and 298) and 2h postprandial (triangles) blood glucose values (n=80 and 104). Mean and standard error (SE) are given.
Mean (standard deviation [SD]) HbA1c level at the start of insulin therapy was 9.4% (1.5) (Fig. 1B). Mean HbA1c levels at one and five years were 7.9% (1.1) and 7.7% (1.2) respectively.
Change over time in insulin treatmentsInsulins most commonly prescribed at the start of the insulin therapy included neutral protamine Hagedorn (NPH) insulin (n=170, 49.1%), premixed human insulin (n=101, 29.2%), and a premixed insulin analog (n=39, 11.3%). After five years, 98 patients (28.3%) were being treated with a premixed insulin analog, while only 44 patients (12.7%) were receiving human basal insulin and 68 patients (19.7%) premixed human insulin.
Changes in insulin treatments were common. Over a five-year period, 42 patients (12.1%) did not change their insulin regimen, while 105 patients (30.4%) changed it once, 90 patients (26%) twice, 54 patients (15.6%) three times, and 55 patients (15.9%) changed it four or more times. Mean (SD) number of changes in insulin treatment per patient was 2.0 (1.5). These results include data from some patients followed up for longer than five years.
Median time from start to the first change in insulin therapy was 25.6 months (n=304), and median time to the second change was 45.5 months (n=119).
Fasting and 2h postprandial blood glucoseMean fasting and 2h postprandial blood glucose levels at the start of insulin therapy were above the recommended control goals of ≤110mg/dL and ≤135mg/dL respectively (Fig. 1A).13 At five years, 51 patients (16.5%) achieved the goal of fasting blood glucose levels ≤110mg/dL and 11 patients (3.6%) achieved the goal of 2h postprandial blood glucose levels ≤135mg/dL.
At the start of insulin therapy, mean (SD) fasting and 2h postprandial blood glucose levels were 223.7 (55.9)mg/dL and 293.6 (71.0)mg/dL respectively (Fig. 1C). One year later, mean fasting and 2h postprandial blood glucose levels were 166.7 (41.0)mg/dL and 225.0 (65.4)mg/dL respectively. Mean fasting and 2h postprandial blood glucose levels at five years were 153 (47.5)mg/dL and 189.1 (47.2)mg/dL respectively.
Complications of diabetesAt the start of the insulin therapy, 110 patients (31.8%) had microvascular complications and 60 patients (17.3%) had macrovascular complications. At least five years later, of the 110 patients with microvascular complications, 62.1% had diabetic retinopathy, 59.8% diabetic nephropathy, 19.5% diabetic neuropathy, and 14.9% diabetic macular edema. On the other hand, of the 60 patients with macrovascular complications, 53.7% had coronary artery disease, 41.5% peripheral artery disease, and 14.6% cerebral artery disease.
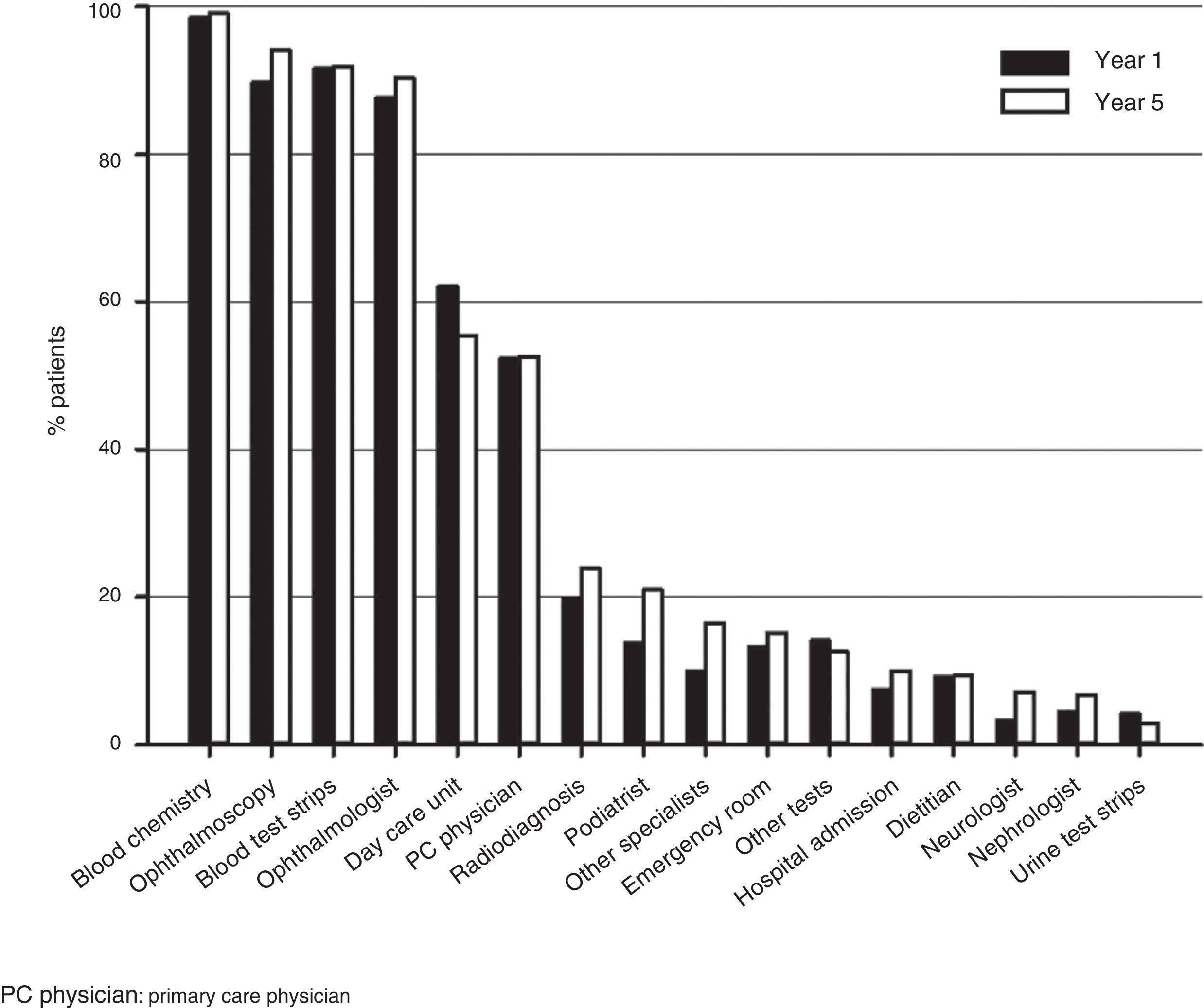
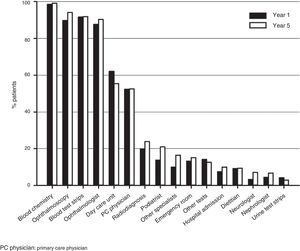
ResourcesThe most commonly used healthcare resources included blood chemistry, ophthalmoscopy, test strips, and visits to ophthalmologist, followed by visits to day care units and primary care physicians (Fig. 2).
DiscussionThis observational, retrospective study assessed the quality of blood glucose control since the start of insulin therapy in clinical practice in Spain, and also characterized patient profile and identified use of healthcare resources by patients on outpatient insulin therapy. At the time this study was designed, our reference for blood glucose control was the European Diabetes Policy Group, which recommended an HbA1c level of ≤6.5%.13 Since then, an HbA1c goal <7.0% has been accepted, with the recommendation to determine the individual treatment goals based on the specific characteristics and comorbidities of each patient.1 Overall blood glucose control improved five years after the start of insulin therapy, as shown by a change in HbA1c from 9.4% at the start of insulin to 7.7% at five years. However, only a limited number of patients achieved an HbA1c level ≤6.5%. Blood glucose levels were high at the start of insulinization and remained high throughout the study. Basal insulin was initially prescribed to half the patients, and several changes in insulin regimen were required during the observation period.
The DCCT and the United Kingdom Prospective Diabetes Study (UKPDS) showed that hyperglycemia is associated to microvascular and macrovascular complications.14,15 In order to minimize the risk of development of complications related to diabetes, the IDF, ADA and EASD issued guidelines for the treatment of type 2 diabetes. The IDF recommends that patients maintain HbA1c levels less than 6.5%,16 and the ADA and EASD consensus recommends levels lower than 7.0%.1 In real clinical practice, however, most patients with type 2 diabetes do not achieve the recommended goals.3
Our data show that insulin therapy was not started until hyperglycemia was markedly high. Cardiovascular risk factors (mean systolic blood pressure 139.3mmHg and mean total cholesterol 220.3mg/dL) and vascular complications (microvascular in 31.8% of the patients and macrovascular in 17.3%) were marked at treatment start, which suggests that patients had metabolic abnormalities for a long time period. At the start of the insulin therapy, almost 10 years after diagnosis on average, mean HbA1c level was 9.4%. These data agree with those from the observational, prospective study INSTIGATE, which assessed standard care for patients with type 2 diabetes in five European countries.11 Patients in the current EDIN study, as those in the INSTIGATE study, started insulin therapy as part of their standard care, and received the standard medical care in the different countries. This study, however, was conducted to better assess care for diabetes throughout Spain. The complete population of the EDIN study, like the Spanish population of the INSTIGATE study,10,11 had elevated, sustained blood glucose levels at the start of insulin therapy and during observations. Patients from the Spanish population of the INSTIGATE study (n=224) started insulin treatment approximately 10 years after diagnosis and had a mean HbA1c level of 9.2%.11,17 Overall, mean HbA1c level at the start of insulin therapy in the INSTIGATE study was 9.6%.11
The fact that HbA1c levels were far above the recommended values at the start of insulin therapy indicates a reality that agrees with previously reported data on diabetes care. In an observational study conducted in Spain, treatment with oral antidiabetics (OADs) was not started until mean HbA1c level exceeded 8%, and OAD treatment was not intensified until two years later, instead of the ideally recommended three months.18 In studies conducted in the United Kingdom, insulin therapy was started on average almost five years after loss of glycemic control, even in the presence of complications related to diabetes,19 and more than 11 years on average after the first prescription of oral treatment.20 The reasons for the delay in starting insulin therapy are diverse and are not always well defined. Reasons may include both patient and physician resistance, although the delay may also be due to the therapeutic paradigm of administering oral hypoglycemic treatment before insulin therapy.21 Whatever the reasons for the delay in insulin therapy in this and other studies, these results suggest an opportunity to improve diabetes care.
Our results show that, after the start of insulin therapy, mean blood glucose level remained quite higher than recommended by international guidelines throughout the study. HbA1c levels were high when changes were made in insulin therapy, including dose intensification. After insulin treatment for at least five years, mean HbA1c level was 7.7%, and only 10.3% of the patients achieved HbA1c values ≤6.5%. These data agree with those from prior studies conducted in Spain, in which approximately 20% of patients with type 2 diabetes had HbA1c levels ≤6.5%,8 and approximately 30% of the patients with type 2 diabetes on insulin therapy had HbA1c levels ≤7.0%.22 The study Cost of Diabetes in Europe-Type II (CODE-2) assessed blood glucose control in patients from Spain and seven other European countries.23 In this study, less than 40% of the Spanish patients and approximately 30% of all patients achieved HbA1c levels <6.5%.23 These data therefore emphasize the need for stricter compliance with the widely accepted recommendations to minimize the risks related to diabetes caused by sustained hyperglycemia.24
This study has limitations and strengths. Limitations include a small sample size, which may have introduced some bias. Patient number was smaller than the planned sample size due to a slower than expected recruitment rate and to the impossibility to extend the study period. The reduced sample size therefore affected accuracy of results. However, the study had adequate statistical power to draw conclusions from the results of this observational design. Long-acting analogs (glargine and detemir) were not available for most of the time period assessed (2000–2007). These observations cannot therefore be extrapolated to the currently available standard treatment. During part of the study period, specialists, rather than primary care physicians, were the main prescribers of the first insulin in patients with type 2 diabetes. There may also have been variability in availability of data about complications in the clinical histories of patients. Strengths of this study include the design to assess under naturalistic conditions patient care at the start of insulin therapy and subsequent results for both patient and disease. While randomized, controlled studies may provide an artificial therapeutic framework for patients, this study provides a perspective of real treatment in Spain.
In conclusion, blood glucose measurements improved in this study after at least five years in patients with type 2 diabetes given insulin therapy. However, mean blood glucose control exceeded the target values recommended by the international community. These results suggest a margin for improving outpatient care in Spain, and the need for considering patient characteristics when individual therapeutic goals are established.
Conflicts of interestFinancial interests of A.R. and J.R.: employees of Eli Lilly and Company. S.T. No conflict of interest reported with regard to this article.
This study was supported by Lilly España, Eli Lilly and Company. The authors thank Simon Cleall, from Eli Lilly and Company, for his critical review of the manuscript, and Joseph Giaconia, from INC Research (Raleigh, NC), for help in manuscript preparation.
Please cite this article as: Rodríguez A, Tofe S, Reviriego J. Evolución clínica a 5 años desde el inicio de la insulinoterapia en pacientes con diabetes tipo 2 en España: resultados del estudio EDIN. Endocrinol Nutr. 2014;61:369–376.