A study of the glycemic control, quality of life, and fear and perception of hypoglycemia by comparing continuous subcutaneous insulin infusion (CSII) group with multiple daily injections (MDI) with bolus calculator group.
Materials and methodsThis is a retrospective cohort study with following up during the first 12 months that CSII group (n=30) begins the use of “bolus wizard” and the MDI-calculator (n=30) group begins the use of the bolus calculator (Accu-Chek® Aviva Expert). Primary outcome: HbA1c (3, 6 and 12 months). Questionnaires used: EsDQOL (quality of life), FH-15 (fear of hypoglycemia), and Clarke (perception of hypoglycemia). Statistical analysis: Student's t and nonparametric tests.
ResultsThe average reduction in HbA1c during the study was significantly higher in CSII group (−0.56±0.84%) compared with the MDI group (0.097±0.94%), p=0.028. The average basal insulin dose was significantly higher in the MDI group (at baseline, 6 and 12 months). No significant differences were found between the 2 treatment groups after analyzing the EsDQOL, FH-15 and Clarke questionnaires. In the CSII group, perceived quality of life assessed by the EsDQOL questionnaire was found to be better at the end of the study than at the beginning of using the insulin pump.
ConclusionsThe average reduction in HbA1c was significantly higher in the CSII group. In the CSII group, perceived quality of life was better at the end of the study than at the beginning.
Comparar el control glucémico, la calidad de vida, así como el miedo y percepción de las hipoglucemias en el grupo de infusión subcutánea continua de insulina (ISCI) frente a la terapia con múltiples dosis de insulina (MDI) con calculador de bolus.
Material y métodosEstudio de cohortes retrospectivo con seguimiento durante los 12 primeros meses desde que el grupo ISCI (n=30) comienza a utilizar el «bolus ayuda» y el grupo MDI-calculador (n=30), comienza a utilizar el calculador de bolus (Accu-Chek® Aviva Expert). Variable de desenlace primaria: HbA1c (3, 6 y 12 meses). Cuestionarios empleados: EsDQOL (calidad de vida), FH-15 (miedo a las hipoglucemias), Clarke (percepción de las hipoglucemias). Análisis estadístico: T de Student y pruebas no paramétricas.
ResultadosEl descenso medio de HbA1c a lo largo del estudio fue significativamente mayor en el grupo ISCI (−0,56±0,84%) que en el grupo MDI (0,097±0,94%), p=0,028. La dosis media de insulina basal fue significativamente mayor en el grupo MDI (al inicio, 6 y 12 meses). No se encontraron diferencias significativas entre ambos grupos de tratamiento en los cuestionarios EsDQOL, FH-15 y Clarke. La calidad de vida percibida por los pacientes, valorada mediante el cuestionario EsDQOL, en el grupo ISCI fue significativamente mejor al final del estudio que al inicio de la utilización de la bomba de insulina.
ConclusionesEl descenso medio de HbA1c fue significativamente mayor en el grupo ISCI. Se objetivó una mejor calidad de vida en el grupo ISCI al final del estudio que al inicio.
Type 1 diabetes mellitus (T1DM) accounts for 5–10% of all cases of diabetes. The publication of the results of the Diabetes Control and Complications Trial1 emphasized the significance of optimizing blood glucose control using an insulin replacement therapy which was as physiological and flexible as possible, such as basal-bolus therapy. There are two possible options for basal-bolus therapy: continuous subcutaneous insulin infusion (CSII) and multiple dose insulin injections (MDI).
The main limiting factors for achieving optimum blood glucose control in intensive T1DM treatment include the fear of hypoglycemia and the lack of efficacy in insulin dose self-adjustment.
Manual calculation of insulin boluses is complex and time-consuming. The use of automated bolus calculators has been shown to be effective in helping insulin pump users to calculate their prandial insulin requirements and to achieve optimum blood glucose control.2
Up to 64% of patients with T1DM make an inadequate calculation of prandial insulin3 because they underestimate their carbohydrate intake.4 Wide postprandial glucose excursions due to inadequate insulin dose adjustment are seen even in patients with good metabolic control.5
Two bolus calculators are currently marketed in Spain, the Accu-Chek® Aviva Expert device from Roche and the FreeStyle InsuLinx®, marketed by Abbott.6 A prospective study7 showed that the most significant decrease in HbA1c occurred in the group using a bolus calculator (−0.8%), but the differences between the groups were not significant. In a survey sent to 1412 patients with T1DM in the United Kingdom regarding their experience using a bolus calculator,8 52% felt that its use had decreased their fear of experiencing hypoglycemia, and 89.3% thought that dose calculation was easier as compared to manual calculation.
In the Diabetes Control and Complications Trial, intensive glucose control was shown to be associated with a three times greater risk of severe hypoglycemia.1 Hypoglycemia unawareness occurs in 20% of patients with T1DM, and is associated with a six times greater risk of severe hypoglycemia.9
Randomized clinical trials comparing treatment with CSII or MDI in T1DM have shown improved HbA1c levels and the potential to decrease the incidence of severe hypoglycemia in the group treated with CSII.10–13
The Clarke et al. questionnaire,14 validated in Spanish, provides information about the perception of hypoglycemia and data about the blood glucose threshold from which symptoms occur. The questionnaire consists of eight questions which have the answers “R” or “A”. Four or more “R” answers suggest a decreased perception of hypoglycemia.
Recently, a Spanish group15 developed a tool called FH-15 to measure the fear of hypoglycemia; this consists of 15 items which are scored using a five-point Likert scale. The cut-off point in the FH-15 scale has been established at 28 points.
The Diabetes Quality of Life, or DQOL, devised by the Diabetes Control and Complications Trial group, has been adapted into Spanish; the Spanish version is called EsDQOL.16 The EsDQOL consists of 43 questions. The total score of each subject is the sum of the scores in each question in the questionnaire. The lower the score, the lesser is the impact of diabetes on the quality of life of the patient.
The main study objective was to assess if differences exist in our environment between patients with T1DM treated with MDI with bolus calculator and patients treated with CSII in the following aspects:
- -
Blood glucose control, measured by HbA1c.
- -
Quality of life, measured using the EsDQOL scale.
- -
Hypoglycemia, measured using the Clarke test, the FH-15 test, and the quantification of severe hypoglycemic episodes requiring health care.
This was a retrospective cohort study comparing blood glucose control, quality of life, and hypoglycemic episodes between the MDI-calculator group (treated with MDI and using a bolus calculator glucometer) and the CSII group.
Study populationThe study population consisted of patients with T1DM monitored at the endocrinology department of the Navarre Hospital Complex. Thirty patients treated with MDI who used a glucometer to calculate boluses (Accu-Chek® Aviva Expert) and 30 patients treated with CSII (Medtronic Paradigm® Veo™) were selected. Patients in the MDI-calculator arm had used the bolus calculator for at least one year (the final date for starting to use the bolus calculator was January 2013). Patients in the CSII arm had started to use the «bolus wizard» within 12 months of the start of CSII therapy. Assignment to the MDI-calculator or the CSII arm was made by non-probabilistic consecutive sampling. Data were collected by clinical interview, physical examination, surveys validated for the Spanish population, and laboratory tests.
Study variablesPrimary outcome measureHbA1c at baseline and 3, 6, and 12 months after the CSII arm started to use the “bolus wizard” and the MDI started to use the bolus calculator of the glucometer.
Secondary outcome measuresThe proportion of patients achieving HbA1c values <7% at 3, 6, and 12 months.
Quality of life: in both treatment groups, the EsDQOL was carried out at the same time, i.e. 12 months after the MDI arm started to use the bolus calculator. In addition, an EsDQOL performed before the start of CSII therapy was available for the CSII arm. Thus, the EsDQOL test performed before the start of CSII therapy and the test performed at the same time as the EsDQOL test was carried out in the MDI-calculator arm were compared in this group only.
A record of severe hypoglycemia and diabetic ketoacidosis episodes requiring health care in both treatment arms was made from 1/01/2013 to 1/01/2014.
Clarke and FH-15 tests: these were performed in both arms at the same time, i.e. 12 months after the MDI arm started to use the bolus calculator.
Weight and basal insulin units/kg of weight: these were analyzed at baseline and at 6 and 12 months.
Data were entered into SPSS® 18.0 statistical software. To compare the means of HbA1c, weight, and the daily dose of basal insulin, the normal distribution of these parameters in the sample was first assessed using a Kolmogorov–Smirnov normality test. When this requirement was met, parametric tests were used, specifically a Student's t test for independent samples. When distribution was not normal, nonparametric tests (Mann–Whitney U test) were used to compare the means of HbA1c, weight, and the daily dose of basal insulin in both treatment groups, using a 95% confidence level. Descriptive statistics (mean, standard deviation, 95% confidence interval [95% CI]) were also calculated for the observed values of HbA1c, the daily dose of basal insulin, weight, etc., in the CSII and MDI arms. A Pearson's Chi-square test was used to compare the proportion of patients who achieved an HbA1c less than 7% in each treatment arm at 3, 6, and 12 months.
Scores in the questionnaires (EsDQOL, Clarke, FH-15) were analyzed globally in order to obtain a single value for each patient and questionnaire. A comparison between the treatment groups was subsequently made for each questionnaire using nonparametric tests. In addition, in the CSII arm, the EsDQOL performed at the start of therapy was compared to the EsDQOL performed at the same time as in the MDI group using nonparametric tests (Wilcoxon).
ResultsTwenty-two patients (36.7%) in the sample were males, and 38 (63.3%) were females. Mean time since diabetes onset was 16.30 years (95% CI: 12.91–19.69) in the CSII arm and 18.13 years (95% CI: 15.38–20.88) in the MDI arm.
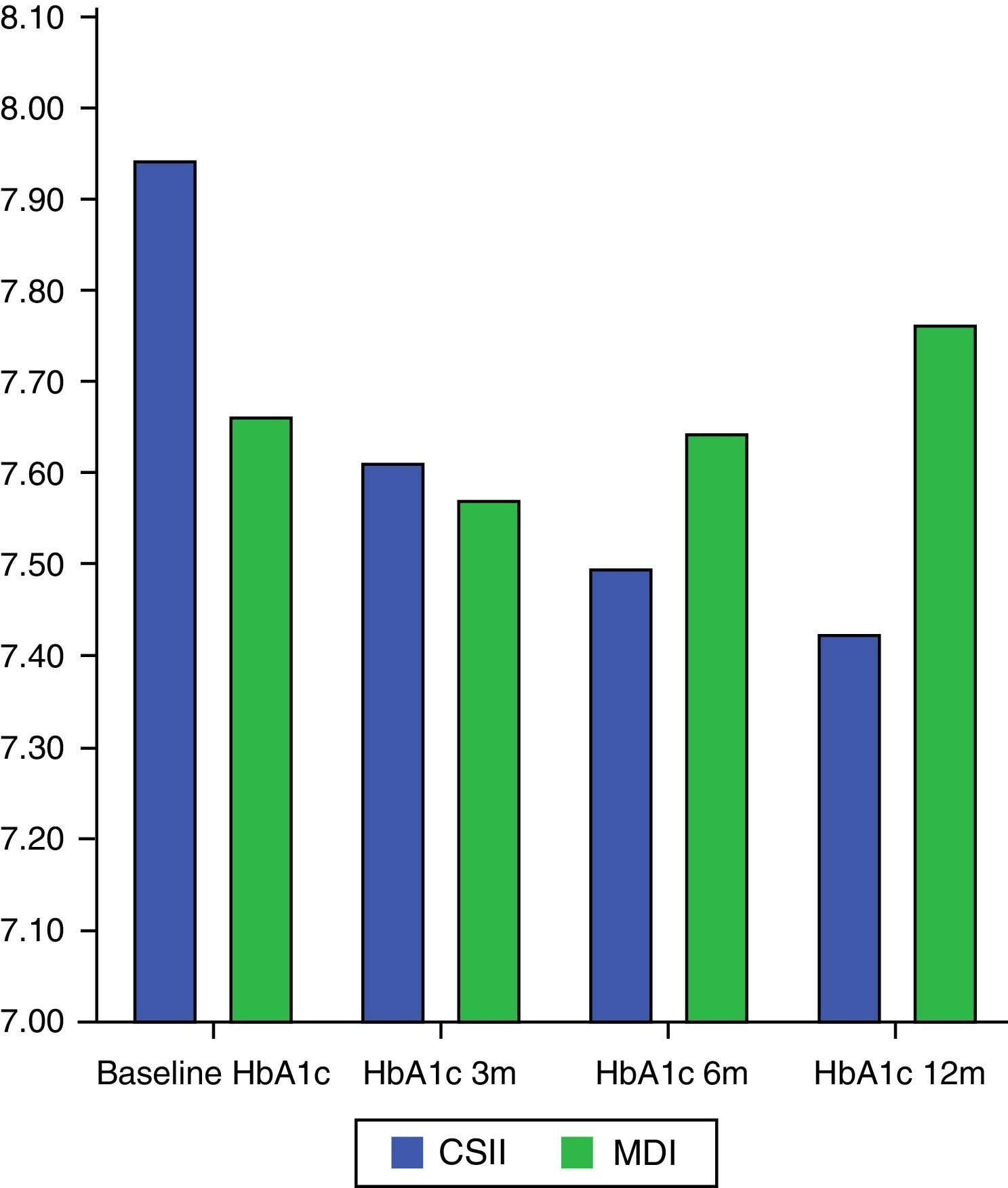
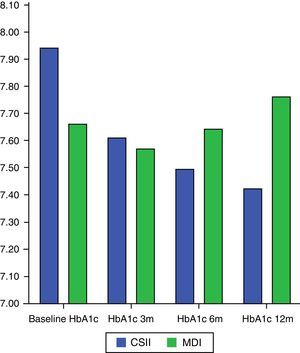
Change over time in HbA1cMean HbA1c decrease during the study (analyzed using a Mann–Whitney U test) was significantly greater in the CSII arm (−0.56±0.84%) as compared to the MDI arm (0.097±0.94%), p=0.028.
In the CSII arm, HbA1c significantly changed during the study (Student's t test for related samples: p=0.001; Wilcoxon test: p=0.003), while no such difference was seen in the MDI arm (Student's t test for related samples: p=0.576; Wilcoxon test: p=0.811) (Fig. 1).
No statistically significant correlation was seen between HbA1c at study start and time since the onset of diabetes (Spearman's rho=−0.155, p=0.240), nor between HbA1c at study end and time since the onset of diabetes (Spearman's rho=−0.085, p=0.519).
An analysis of the proportion of patients who achieved HbA1c<7% in each arm showed that 21.4% (6 patients) in the CSII arm and 24.1% (7 patients) in the MDI group (p=0.807) reached the goal at 3 months. At 6 months, the goal was achieved by 34.5% (10 patients) in the CSII arm and 20.7% (6 patients) in the MDI arm (p=0.240). Finally, the glucose control goal was achieved at 12 months by 33.3% (10 patients) in the CSII arm and by 26.7% (8 patients) in the MDI arm (p=0.573).
Change over time in weightA separate analysis of the groups showed that statistically significant weight changes occurred during follow-up in the CSII arm (Student's t test for related samples: p=0.016, Wilcoxon test: p=0.014), but not in the MDI arm (Student's t test for related samples: p=0.834; Wilcoxon test: p=0.188). Weight increase during the study (analyzed using a Mann–Whitney U test) was 2.11±4.53kg in the CSII arm and 0.16±4.22kg in the MDI arm (p=0.209).
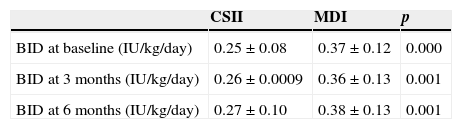
Change over time in mean dose of basal insulinMean basal insulin dose (IU/kg/day) was significantly higher in the MDI arm (at baseline and at 6 and 12 months) (Table 1). No statistically significant correlation was found between basal insulin dose at 12 months and weight at 12 months (Spearman's rho=0.139, p=0.299).
Summary of basal insulin dose.
| CSII | MDI | p | |
|---|---|---|---|
| BID at baseline (IU/kg/day) | 0.25±0.08 | 0.37±0.12 | 0.000 |
| BID at 3 months (IU/kg/day) | 0.26±0.0009 | 0.36±0.13 | 0.001 |
| BID at 6 months (IU/kg/day) | 0.27±0.10 | 0.38±0.13 | 0.001 |
BID, basal insulin dose; CSII, continuous subcutaneous insulin infusion; MDI, multiple dose insulin injections.
Two patients (6.7%) in the CSII arm and one patient (3.3%) in the MDI arm had an episode of diabetic ketoacidosis.
On the other hand, no severe hypoglycemic episode occurred in the CSII arm, while one patient (3.3%) in the MDI group experienced an episode of severe hypoglycemia during follow-up.
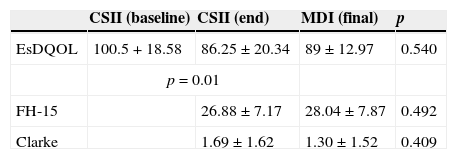
EsDQOL, FH-15, and Clarke questionnairesOverall, 76.7% of patients completed the questionnaires (86.7% in the CSII arm and 66.7% in the MDI arm). Table 2 summarizes the results.
Summary of the EsDQOL, FH-15, and Clarke questionnaires.
| CSII (baseline) | CSII (end) | MDI (final) | p | |
|---|---|---|---|---|
| EsDQOL | 100.5+18.58 | 86.25±20.34 | 89±12.97 | 0.540 |
| p=0.01 | ||||
| FH-15 | 26.88±7.17 | 28.04±7.87 | 0.492 | |
| Clarke | 1.69±1.62 | 1.30±1.52 | 0.409 | |
CSII, continuous subcutaneous insulin infusion; MDI, multiple dose insulin injections. Bold: The score in the EsDQOL questionnaire of the CSII arm when insulin pump use was started was significantly higher (Wilcoxon test, p=0.010) than the score in the questionnaire performed at the same timepoint as in the MDI arm.
A comparison of the EsDQOL score of the CSII arm at the start of the use of the insulin pump and the score found when the questionnaire was completed at the same time as in the MDI arm showed a significantly lower mean at study end as compared to the start of the use of the insulin pump, 86.25±20.34 versus 100.50±18.5 (Wilcoxon test, p=0.010).
However, differences in EsDQOL scores between both treatment groups were not statistically significant (p=0.540). When EsDQOL was compared by sex using nonparametric tests (Mann–Whitney U test), mean values of 78.63±19.91 and 86.25±20.34 were found in males and females respectively (p=0.244).
FH-15The CSII arm had a lower mean score (less fear of hypoglycemia) than the MDI arm in the FH-15 questionnaire: 26.88±7.17 and 28.04±7.87 respectively) (p=0.492).
A score of 28 points or higher in this questionnaire allowed us to classify patients as having a fear of hypoglycemia. In both the CSII and MDI arms, 9 patients (30%) had a score of 28 points of higher and so could be considered as having a fear of hypoglycemia.
A weak to moderate correlation existed between time since diabetes onset and the FH-15 questionnaire (rho=0.464, p=0.001), so that a longer time correlated to a higher score in the FH-15 test for a fear of hypoglycemia.
ClarkeIn the Clarke questionnaire for detecting inadvertent hypoglycemia, the MDI arm had a lower score, 1.30±1.52 versus 1.69±1.62 (p=0.409).
The cut-off point in the Clarke questionnaire is 4, four or more “R” answers suggesting a decreased perception of hypoglycemia. Six patients (20%) in the CSII arm had a score in the Clarke scale of 4 of higher, so that 20% of patients in the CSII arm had a decreased perception of hypoglycemia. By contrast, only two patients (6.7%) in the MDI had a decreased perception of hypoglycemia.
There was a weak, non-statistically significant correlation (rho=0.191, p=0.209) between the time since diabetes onset and the score achieved in the Clarke test.
DiscussionOur retrospective cohort study showed that treatment with CSII, as compared to MDI using a bolus calculator, achieved better blood glucose control, as measured by a decrease in HbA1c at one year of treatment. In fact, a mean decrease by 0.56% in HbA1c occurred in the CSII arm (p=0.028) during the study.
This finding is consistent with a Cochrane review17 comparing treatment with CSII and MDI in which 23 randomized, controlled clinical trials involving 976 patients with T1DM were analyzed. The difference in HbA1c means in this review was −0.3% (95% CI: −0.1 to −0.4) in favor of CSII therapy.
Our study results also agree with those of another meta-analysis18 including 11 randomized, controlled clinical trials comparing CSII and MDI in T1DM. In this meta-analysis, CSII therapy was associated with a significant improvement in HbA1c as compared to MDI, with a standardized mean difference of −0.3% (95% CI, −0.4 to −0.1, p<0.001). Similar results were reported in other randomized, controlled clinical trials comparing treatment with CSII or MDI in T1DM, in which an improvement in HbA1c was found in the group treated with CSII.10–13,19
As regards weight, a significant change occurred in the CSII arm during our study. Weight differences seen in our sample did not correlate to differences in basal insulin dose in each treatment group, as no statistically significant correlation was seen between weight and basal insulin dose at the end of the study.
An analysis of mean basal insulin dose in both treatment groups showed significantly lower basal insulin requirements in the CSII arm at baseline and after 6 and 12 months of treatment. These findings are similar to those of the Cochrane review,17 where the difference in mean daily insulin requirements was −7IU (95% CI: −11 to −3) in favor of CSII as compared to MDI (p=0.0003).
No patient in the CSII arm experienced severe hypoglycemic episodes (requiring health care), while one patient (3.3%) in the MDI group had severe hypoglycemia. In the Cochrane review,17 the 15 studies reporting severe hypoglycemic episodes used different scales; however, data suggest that CSII may be a better therapy than MDI as regards reducing the incidence of severe hypoglycemic events. The review also agrees with the results reported in another meta-analysis20 of 22 studies aimed at comparing severe hypoglycemic episodes in the MDI and CSII groups. A total of 1414 patients with T1DM were compared during a median duration of CSII therapy of 6–48 months. This meta-analysis found a significantly greater reduction in severe hypoglycemic episodes with CSII as compared to MDI. Moreover, the reduction in severe hypoglycemic episodes was greater in patients with a greater frequency of hypoglycemia at study start, and mean age was found to be a clear predictor of the effect of treatment (p=0.019), with older subjects showing a greater reduction in severe hypoglycemic episodes.
As regards diabetic ketoacidosis episodes, a greater predisposition to them was seen in the CSII arm (6.7% versus 3.3% in the MDI arm), probably because of the lower subcutaneous insulin deposit in this type of therapy.
The EsDQOL questionnaire showed a greater quality of life in the CSII arm at the end of the study as compared to the start of the use of the insulin pump (Wilcoxon test, p=0.010). However, no significant differences were seen in our study between the treatment groups, although the CSII arm had a trend to higher scores, reflecting a better quality of life. In the Cochrane review,17 quality of life was measured using different tools in 15 studies, but the data suggest that most participants were more satisfied with CSII. When quality of life was analyzed by sex in our study, males showed lower scores (p=0.244). These results agree with those reported in other studies21,22 in which females were more concerned about diabetes than males.
In the Clarke test, 20% of patients in the CSII arm and only 6.7% in the MDI arm had a decreased perception of hypoglycemia. This result confirms that recurrent severe, nocturnal, or inadvertent hypoglycemia is one of the main indications for CSII therapy.23
The main limitation of this study is that it was a retrospective cohort study. Ideally, the best design for our study would have been a randomized, even crossover, clinical trial. However, by comparing these two types of advanced therapies for patients with T1DM, we have been able to draw preliminary conclusions regarding the design of future studies. An additional study limitation is that the EsDQOL questionnaire was not analyzed in subjects from the MDI arm before the start of the bolus calculator, so that the results suggesting the quality of life improvement recorded in the treatment groups are poorly comparable.
Ideally, the best comparison groups for our study would have been patients who started to use CSII and the “bolus wizard” at the same time versus patients in the MDI-calculator arm. However, because of logistic problems, the CSII arm enrolled patients who had started to use the “bolus wizard” within 12 months of CSII start in order to try to ensure that variables such as weight or HbA1c were affected as little as possible and thus to be able to have two more comparable treatment groups. However, this may have limited our study results. On the other hand, it should be noted that the two populations can never be fully comparable because of the selection bias involved in the clearly established indications for CSII therapy.
The main practical application of this study comes from the obtaining of comparative data regarding the main types of intensive therapy for T1DM currently available after the introduction of the bolus calculator in clinical practice.
ConclusionsA significantly greater mean decrease in HbA1c occurred in the CSII arm during the study. Mean basal insulin doses were significantly higher in the MDI arm. Quality of life in the CSII arm was better at the end of the study than at the start of the use of the insulin pump. In addition, a longer time since diabetes onset correlated to a higher score in the test for the fear of hypoglycemia. New studies (prospective, randomized) are needed to assess whether relevant differences exist between CSII (with the bolus wizard system) and MDI with a bolus calculator in terms of blood glucose control, quality of life, and hypoglycemic episodes.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Pérez-García L, Goñi-Iriarte MJ, García-Mouriz M. Comparación del tratamiento con infusión subcutánea continua de insulina frente a la terapia con múltiples dosis de insulina con calculador de bolus en pacientes con diabetes tipo 1. Endocrinol Nutr. 2015;62:331–337.