Medullary thyroid cancer is a rare tumor that is more aggressive and has a worse prognosis than differentiated thyroid cancer. The purpose of this study was to report the demographic, clinical, and genetic characteristics of patients seen in the health care system of the community of Castilla-La Mancha over a 16-year period.
Patients and methodsData were collected through a review of patients’ medical records.
ResultsThe medical records of 58 patients (mean age at diagnosis, 51 years; range 6–82 years; 63.8% women) were reviewed. Prevalence rate was 2.84 cases per 100,000 inhabitants, with a high variability between areas (range 0–5.4 cases per 100,000 inhabitants). Familial cases accounted for 34.5% of all medullary thyroid cancers, and the most common mutation was C634Y. The condition was most commonly diagnosed following palpation of a cervical lump (70.6%). At diagnosis, 56 of 58 patients underwent ultrasound and 8 of 58 patients were tested for serum calcitonin. Tumor multicentricity was reported in 59 and 50% of patients with multiple endocrine neoplasia syndrome type 2A and 2B, respectively, and in no sporadic cases. Fifty-two percent of patients had an advanced stage (III or IV) at diagnosis. Median follow-up was 36 months (interquartile range 14–210); 11 patients were lost to follow-up.
ConclusionsIn Castilla-La Mancha, medullary thyroid cancer is diagnosed by cervical ultrasound, rather than calcitonin assay. There is a high prevalence of both familial and sporadic medullary thyroid cancer, and a significant variability in the type of proto-oncogen rearranged during transfection mutation as compared to the rest of the Spanish population.
El carcinoma medular de tiroides es un tumor de baja prevalencia cuyo pronóstico es peor que el del cáncer diferenciado de tiroides debido su mayor agresividad. El objetivo de este trabajo es describir las características demográficas, clínicas y genéticas de los pacientes atendidos en el área sanitaria de la Comunidad de Castilla-La Mancha durante 16 años.
Pacientes y métodosLos datos se recogieron mediante revisión de historias clínicas.
ResultadosSe revisaron las historias clínicas de 58 pacientes con una edad media al diagnóstico de 51 años (intervalo de 6 a 82 años) y un 63,8% de mujeres. La prevalencia fue de 2,84 casos por 100.000 habitantes, con una gran variabilidad entre áreas (de 0 a 5,4 casos por 100.000 habitantes). Los casos familiares representaron el 34,5% del total, siendo la mutación más frecuente la C634Y. El motivo más frecuente de diagnóstico fue la palpación de un bultoma cervical (70,6%); se solicitó ecografía al diagnóstico en 56 de 58 casos, y la calcitonina en 8 de 58 casos. La multicentricidad del tumor fue descrita en el 59 y 50% de los casos de síndrome de neoplasia endocrina múltiple tipo 2A y 2B, respectivamente, y en ningún caso esporádico. El 52% de los pacientes presentaba un estadio avanzado al diagnóstico (iii o iv). La mediana de seguimiento fue de 36 meses (rango intercuartílico 14-210), con la pérdida de 11 pacientes durante el seguimiento.
ConclusionesEl diagnóstico de carcinoma medular de tiroides en Castilla-La Mancha se basa en la ecografía cervical, pero no en la calcitonina. Existe una alta prevalencia de este carcinoma, tanto familiar como esporádico, y una importante variabilidad en el tipo de mutación del protooncogén rearranged during transfection comparadas con las del resto de la población española.
Medullary thyroid cancer (MTC) is a rare tumor accounting for 5%–10% of all thyroid cancers.1 MTC arises from parafollicular or C cells in the thyroid gland and causes calcitonin hypersecretion as early biochemical signal. It may be sporadic or be part of the multiple endocrine neoplasia type 2 (MEN-2) syndrome, which has an autosomal dominant inheritance pattern. MTC easily invades intraglandular lymph nodes and expands to the rest of the thyroid gland, and to pericapsular and regional lymph nodes. Early diagnosis is associated to better prognosis.2 Because of the low prevalence of this condition, analysis of all diagnosed cases is essential to increase its understanding. In this regard, this study first provides information about the incidence of MTC in Castile-La Mancha.
The purpose of this study was to report the demographic and clinical characteristics of a series of patients with MTC seen at the eight healthcare areas of the community of Castile-La Mancha, and to analyze the differences between sporadic and familial cases.
Patients and methodsAn observational, retrospective, multicenter study sponsored by the Castile-La Manche Society of Endocrinology, Nutrition and Diabetes was conducted in all patients with pathological diagnosis of MTC between 1995 and 2010, inclusive. Patients with pathological diagnosis of C-cell hyperplasia were excluded from this study. The databases of the pathology departments of the eight healthcare areas of Castile-La Mancha (Albacete, Ciudad Real, Cuenca, Guadalajara, Mancha-Centro, Puertollano, Talavera de la Reina, and Toledo) were searched for all cases where cytological or histological diagnosis included the term «medullary thyroid cancer”. The Manzanares hospital did not participate in the study. Data were collected from January to September 2011 by reviewing the clinical records of patients previously selected.
The study was approved by the ethics committee and the clinical research committee of the Toledo Hospital Complex, which acted as coordinating center for the study.
Variables analyzedThe following variables were collected for each patient:
- 1.
Demographic variables: age, sex, and reference healthcare area.
- 2.
Clinical variables: age at diagnosis, reason for diagnosis, follow-up time in months, imaging tests requested for diagnosis, calcitonin measurement at diagnosis (yes/no), multicentricity in the surgical specimen (yes/no), presence and location of metastases, TNM stage at diagnosis, and treatment performed (surgical and/or medical).
- 3.
Genetic study: phenotype-genotype classification and type of mutation in the rearranged during transfection (RET) gene, if present. Cases reported as sporadic where those with no mutation in the RET gene.
Prevalence was estimated based on population data from the DIACAM1 study, which were reported in a prior publication.3 Distant metastases were documented based on the clinical records and imaging tests.
Ultrasonographic, cytological, and pathological characteristics of tumors were not analyzed in this study, except for multicentricity in the surgical specimen.
Statistical analysisQuantitative variables are given as the mean and standard deviation if normally distributed, or as the median and range or interquartile range (IR) if not normally distributed. Qualitative variables are given as percentages. Parametric tests used included a Student's t test for means comparison and a Chi-square test to compare qualitative variables. A value of p<0.05 was considered statistically significant. Data analysis was performed using the statistical package SPSS® v.11.0.
ResultsThe clinical histories of 58 patients diagnosed with MTC between 1995 and 2010 were reviewed. Two patients underwent no surgery due to the presence of locally advanced disease. These two cases were diagnosed by cytology with fine needle aspiration. All other cases were diagnosed by histological examination of surgical specimens.
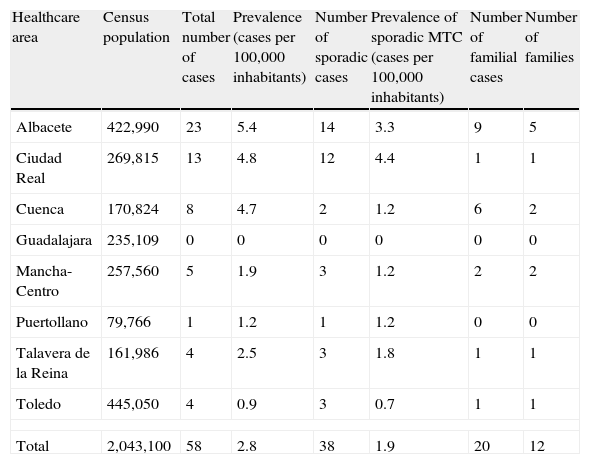
PrevalenceMean prevalence of MTC in our cohort was 2.84 cases per 100,000 inhabitants, with a great variability in the different healthcare areas for both sporadic cases (4.4 and 3.3 cases/100,000 population in the Ciudad Real and Albacete areas, as compared to 0 and 0.7cases/100,000 population in the Guadalajara and Toledo areas) and familial cases (9 cases in 5 families in the Albacete area versus no familial cases in the Guadalajara or Puertollano areas; Table 1).
Prevalence of medullary thyroid cancer.
| Healthcare area | Census population | Total number of cases | Prevalence (cases per 100,000 inhabitants) | Number of sporadic cases | Prevalence of sporadic MTC (cases per 100,000 inhabitants) | Number of familial cases | Number of families |
| Albacete | 422,990 | 23 | 5.4 | 14 | 3.3 | 9 | 5 |
| Ciudad Real | 269,815 | 13 | 4.8 | 12 | 4.4 | 1 | 1 |
| Cuenca | 170,824 | 8 | 4.7 | 2 | 1.2 | 6 | 2 |
| Guadalajara | 235,109 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mancha-Centro | 257,560 | 5 | 1.9 | 3 | 1.2 | 2 | 2 |
| Puertollano | 79,766 | 1 | 1.2 | 1 | 1.2 | 0 | 0 |
| Talavera de la Reina | 161,986 | 4 | 2.5 | 3 | 1.8 | 1 | 1 |
| Toledo | 445,050 | 4 | 0.9 | 3 | 0.7 | 1 | 1 |
| Total | 2,043,100 | 58 | 2.8 | 38 | 1.9 | 20 | 12 |
MTC: medullary thyroid cancer.
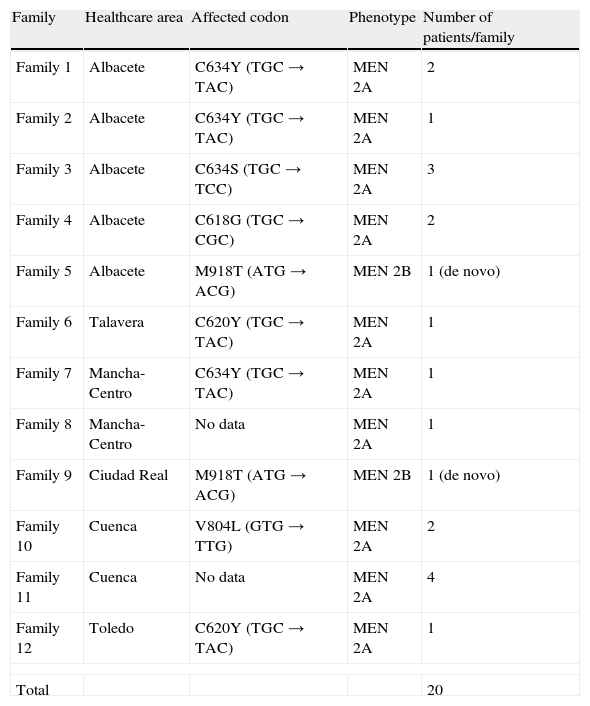
63.8% were females, with a male/female ratio of 1/1.76. Mean age at diagnosis was 50.82 years (range 6–82 years) with no statistical difference between sexes. The ratio between sporadic and familial cases was 38/20. Familial cases represented 34.5% of all patients analyzed (18 patients MEN 2A and 2 patients MEN 2B). The C634Y and C620Y mutations (found in 3 and 2 out of 9 families respectively) were most common (Table 2).
Genotype-phenotype in cases of familial medullary thyroid cancer.
| Family | Healthcare area | Affected codon | Phenotype | Number of patients/family |
| Family 1 | Albacete | C634Y (TGC→TAC) | MEN 2A | 2 |
| Family 2 | Albacete | C634Y (TGC→TAC) | MEN 2A | 1 |
| Family 3 | Albacete | C634S (TGC→TCC) | MEN 2A | 3 |
| Family 4 | Albacete | C618G (TGC→CGC) | MEN 2A | 2 |
| Family 5 | Albacete | M918T (ATG→ACG) | MEN 2B | 1 (de novo) |
| Family 6 | Talavera | C620Y (TGC→TAC) | MEN 2A | 1 |
| Family 7 | Mancha-Centro | C634Y (TGC→TAC) | MEN 2A | 1 |
| Family 8 | Mancha-Centro | No data | MEN 2A | 1 |
| Family 9 | Ciudad Real | M918T (ATG→ACG) | MEN 2B | 1 (de novo) |
| Family 10 | Cuenca | V804L (GTG→TTG) | MEN 2A | 2 |
| Family 11 | Cuenca | No data | MEN 2A | 4 |
| Family 12 | Toledo | C620Y (TGC→TAC) | MEN 2A | 1 |
| Total | 20 | |||
MEN 2A: multiple endocrine neoplasia type 2A syndrome; MEN 2B: multiple endocrine neoplasia type 2B syndrome.
Age at diagnosis of sporadic cases, MEN 2A, and MEN 2B was 54 (range 14–82 years), 40 (range 6–75 years), and 19 years (range 18–21 years) respectively, with a statistically significant difference (p<0.05). In the MEN 2A group, exclusion from analysis of the only pediatric patient did not change statistical significance between the groups; mean age of the MEN 2A after excluding the 6-year-old patient was 46 years (range 21–75 years).
The most common reason leading to MTC diagnosis was pathological neck palpation (thyroid nodule or adenopathy) in 41 patients (70.6%), followed by genetic study, performed on relatives of people with hereditary MTC, in 6 patients (10.3%). Other reasons for diagnosis were incidental finding of a tumor in ultrasound examination (4 patients), study of distant metastases (4 patients), incidental finding at pathological examination after thyroidectomy (2 patients), and workup for ectopic ACTH secretion (one patient).
Laboratory, imaging, and pathological findingsSerum calcitonin was measured before surgery in 8 of the 58 patients, six of them first-degree relatives of a patient diagnosed with MEN. As regards imaging tests, ultrasound examination was most commonly requested for diagnosis (56 of 58 patients).
Multicentricity was found in the surgical specimen in 59% of MEN 2A cases analyzed (10 out of 17 patients, one patient with no data), 50% of cases of MEN 2B (one of two patients), and no sporadic case. The difference was statistically significant (p<0.001).
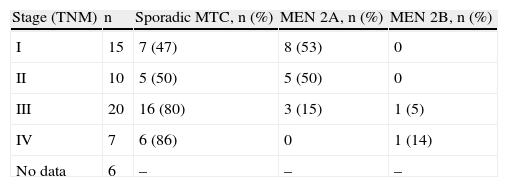
StagingAt diagnosis, 7 patients had stage V disease (2 with liver metastases, 2 with bone metastases, 1 with lung metastases, and 2 with locally advanced disease). Six of them had sporadic forms of MTC, while one patient had MTC associated to MEN 2B. Of the 20 cases with stage III MTC, 15 were sporadic, 4 were associated to MEN 2A, and one was associated to MEN 2B. Among the 25 cases with stage I and II disease, 12 were sporadic forms and 13 were associated to MEN 2A. No data were available for 5 patients (Table 3).
Stage at diagnosis.
| Stage (TNM) | n | Sporadic MTC, n (%) | MEN 2A, n (%) | MEN 2B, n (%) |
| I | 15 | 7 (47) | 8 (53) | 0 |
| II | 10 | 5 (50) | 5 (50) | 0 |
| III | 20 | 16 (80) | 3 (15) | 1 (5) |
| IV | 7 | 6 (86) | 0 | 1 (14) |
| No data | 6 | – | – | – |
MEN 2A: multiple endocrine neoplasia type 2A syndrome; MEN 2B: multiple endocrine neoplasia type 2B syndrome.
The two patients who did not undergo surgery because of advanced disease died from MTC within one year of diagnosis. Surgery was performed in all other patients, but no data are available on the procedure used or on whether lymph node dissection was performed or not.
No follow-up data were available for 11 patients. The median follow-up time of the remaining 47 patients was 36 months (IR, 14–210 months). Seven of these 47 patients died from MTC (median follow-up, 68.5 months; IR, 24–152 months); one patient died from a cause unrelated to MTC; 29 patients were free of disease at the control visit (median follow-up, 36 months; IR, 6–210 months); 8 patients had persistent disease with no distant metastases (median follow-up, 33 months; range 4–192 months), and 4 patients had persistent disease with distant metastases (median follow-up, 45.5 months; range 12–50 months).
DiscussionThis study confirmed many of the demographic characteristics reported in prior publications of series of patients with MTC.4 Mean age at diagnosis was approximately 50 years (51 years), as occurred in most studies reported. However, the proportion of females (65%) was higher than previously reported. The younger age at diagnosis in hereditary cases was also supported, probably because of early detection in relatives of patients with MEN 2A, which is associated to better prognosis.5 Inclusion of a single patient of pediatric age (6 years) was possibly due to performance of prophylactic thyroidectomy in most pediatric cases detected by genetic testing and, thus, not included in this analysis. MTC associated to MEN 2B occurs at a younger age and is associated to greater aggressiveness and poorer prognosis.6
As regards prevalence of hereditary cases, it was higher than expected (35%), but widely variable prevalence rates have been reported, reaching up to 65% depending on the region studied. The proximity of the region of Murcia, where a greater prevalence of familial forms has been reported, may explain the greater presence of familial forms in the Albacete healthcare area (5 of the 11 families in the community of Castile-La Mancha).7
Once detailed the type of mutation in the RET proto-oncogene, it should be noted that the C634R mutation is most common worldwide, occurring in 52% of cases, according to the International RET Mutation Consortium Study.8 In Spain, however, the C634Y mutation is most common, as reported in the Sánchez et al.9 study, which found it in 73% of cases. Regional studies conducted in Spain support this finding. Thus, for example, the reported prevalence of that mutation in the population from Murcia is up to 80%.10 This led to hypothesize the existence of a common ancestor to explain most cases occurring in the Spanish population. In Castile-La Mancha, however, this would only explain 4 out of 15 cases and account for 3 out of 9 families. This is a striking fact, because although the C634Y mutation was most commonly found in the Castile-La Mancha healthcare area, it only accounted for 26% of cases, instead of the 73% reported for the Spanish population,9 and this study found a significant variability in the type of mutation of the RET proto-oncogene, which appears to be an exception as compared to the rest of Spain.
As regards tumor multicentricity, this was a pathognomonic finding of hereditary MTC in our series. However, data reported to date suggest the presence of multiple foci of MTC in sporadic cases also, with their proportion being lower as compared to familial cases, but variable depending on the series, up to 53% of sporadic cases.10 The diagnostic criterion of multicentricity would therefore have to be standardized in our area to achieve a more accurate histological diagnosis.
Analysis of stage at diagnosis in our series showed that, in agreement with data from other series,4,11,12 approximately half the patients were in an advanced stage (III or IV), which is associated to poorer prognosis.13 In this regard, routine calcitonin measurement in thyroid nodule management is controversial.2 Calcitonin levels at diagnosis were measured in 8 of 58 patients because they were relatives of patients with hereditary MTC or had a cytological diagnosis of MTC. It can therefore be stated that calcitonin measurement is not routinely requested in our area for thyroid nodule management, while thyroid ultrasound examination is almost universally used (it had been performed in all but two of our patients). Although data reported in recent years on routine calcitonin measurement appear to suggest that this would be a cost-effective test, there is no universal agreement on systematic calcitonin detection for the study of thyroid nodule. Specifically, while the European consensus for the management of differentiated thyroid cancer supports calcitonin measurement14 and the American Thyroid Association (ATA) guidelines on management of thyroid nodule and differentiated thyroid cancer2 do not recommend or advise against the test, the American Thyroid Association guidelines on management of medullary thyroid cancer15 advise against routine calcitonin measurement. It would be interesting to conduct a cost-effectiveness study in our environment to better define the value of this test in the initial workup of thyroid nodule.
Potential study biases include design, because a retrospective study does not guarantee inclusion of all cases of MTC occurring in Castile-La Mancha, which would explain the disparity in prevalence between the regions. An additional explanation of such variability may be the proximity of the community of Madrid, which may attract patients from neighboring provinces because of its traditional condition of reference center. This may explain the low prevalence of MTC in the provinces of Toledo and Guadalajara. On the other hand, the nearness of the Albacete area to the community of Murcia may justify the higher prevalence of familial forms in this healthcare area.
Another study limitation lies in loss to follow-up of several patients, which decreases accuracy of the reported data.
In conclusion, it may be stated that diagnostic management of thyroid nodule in our setting is based on neck ultrasound examination, with no routine calcitonin measurement. Special characteristics of our series included the high proportion of familial cases, the variability in genotype of the RET proto-oncogene as compared to the remaining Spanish population, and the heterogeneity of MTC prevalence in the different healthcare areas. The less advanced stage of familial cases, excluding MEN 2B, reminds us of the importance of early diagnosis and the need for optimizing diagnosis to improve prognosis of MTC.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Louhibi L, Marco A, Pinés PJ, Padillo JC, Gómez I, Valero MA, et al. Demografía, características clínicas y genéticas de pacientes con carcinoma medular de tiroides en los últimos 16 años en Castilla-La Mancha. Endocrinol Nutr. 2014;61:398–403.