Hyperthyroidism is caused by inadequate thyroid hormone synthesis and/or secretion due to different causes. The prevalence of hyperthyroidism is approximately 1%. The most common cause of thyrotoxicosis is primary autoimmune hyperthyroidism (Graves’ disease), in which stimulation by anti-TSH receptor antibodies (TRAb) causes an increased production of thyroid hormones. In Europe, thioamides are the treatment of choice for Graves’ disease, while radioiodine therapy and surgery are considered as second line treatments. Methimazole, like carbimazole and propylthiouracyl, is a member of the thioamide class, and is one of the main drugs used to treat hyperthyroidism.1
As with any other drug, adverse effects of different severity have been reported for antithyroid drugs. The most common and mildest adverse effects include skin reactions (4–6%), joint pain (1–5%), and gastrointestinal changes (1–5%). The most commonly reported serious adverse effects include agranulocytosis (0.1–0.5%) and hepatotoxicity (0.1–0.2%). The abovementioned mild changes are assumed to be dose-dependent with methimazole and carbimazole, while the hepatotoxicity of these two drugs, unlike as occurs with propylthiouracyl, is idiosyncratic.2–5
The prevalence of acute hepatitis induced by antithyroid drugs ranges from 0.1% to 1%, and most cases reported in the literature were due to propylthiouracyl. Data from 2003 reported a total of 83 cases of acute hepatitis induced by propylthiouracyl, although it has been suggested that the condition may occur in up to 1.2% of patients treated with propylthiouracyl.3,7
To date, less than 40 cases of acute hepatitis secondary to methimazole or carbimazole have been reported in the literature.6 Acute cholestatic hepatitis4–6 was reported in most cases, but there was a case each with a predominant cytolysis pattern,8 granulomatous hepatitis,9 and hepatic steatosis.10
Case reportWe report the case of a 68-year-old male, a former smoker with no alcohol consumption and a history of high blood pressure, dyslipidemia, ischemic heart disease, and secondary dilated cardiomyopathy. The patient had had a pacemaker implanted in 2008 for a bradycardia-tachycardia syndrome. Since then, he had experienced multiple episodes of atrial flutter and fibrillation (AF), which led to treatment being started with amiodarone 200mg/day three years before the start of the condition. His usual treatment included: bisoprolol 5mg, olmesartan 20mg, fluvastatin 80mg, ezetimibe 10mg, acetyl salicylic acid 100mg, nitroglycerin patches 5mg, acenocoumarol 1mg, omeprazole 20mg, and escitalopram 15mg.
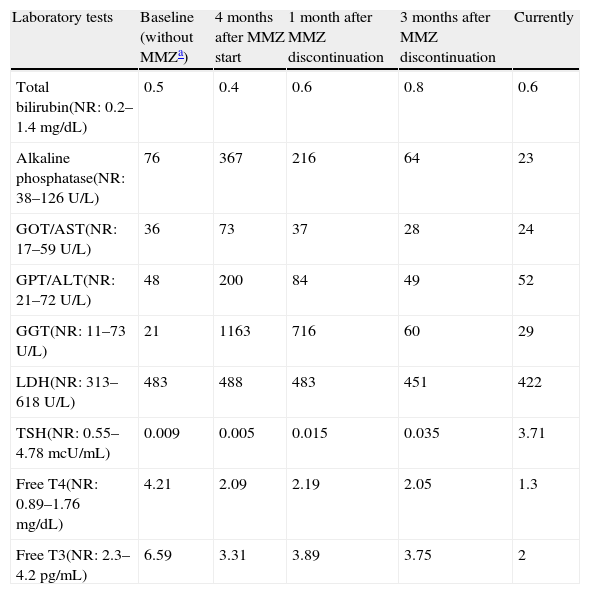
During a hospital admission in May 2011 for a new atrial fibrillation episode, the endocrinology department was consulted because of findings consistent with primary hyperthyroidism: FT4 4.21ng/dL (0.89–1.76), FT3 6.59pg/mL (2.3–4.2), TSH 0.009mcU/ml (0.55–4.78). When questioned, the patient did not report other symptoms suggesting thyroid hyperfunction. Palpation of the anterior neck region was unremarkable, as was the rest of the physical examination. Autoimmune thyroid tests showed anti-TPO antibody levels of 60.6U/mL (0–60), anti-thyroglobulin levels of 213U/mL (0–60), and negative anti-TSI antibodies. A thyroid ultrasound showed no significant changes. Type 2 versus mixed hyperthyroidism secondary to amiodarone was suspected, and treatment was therefore started with prednisone 20mg daily and methimazole 10mg every 8h. The dose of both drugs was doubled due to lack of response (FT4 5.23ng/dL, FT3 6.15pg/mL, TSH 0.015mcU/mL) and a new AF episode with rapid average ventricular response two months later. One month later, laboratory tests provided the following results: total bilirubin 0.4mg/dL (normal 0.2–1.4), GOT/AST 73U/L (normal 17–59), GPT/ALT 200U/L (21–72), GGT 1163U/L (11–73), alkaline phosphatase 367U/L (38–126), LDH 598U/L (313–618). These results were consistent with a pattern of dissociated cholestasis which was not present in the tests performed in May 2011 (total bilirubin 0.5mg/dL, GOT/AST 36U/L, GPT/ALT 48U/L, GGT 21U/L, alkaline phosphatase 76U/L, LDH in blood 483U/L).
Because of the recent start of treatment with methimazole and since no other immediate causes explaining such laboratory changes were found, acute hepatitis probably induced by this drug was considered, and antithyroid drugs were therefore immediately discontinued. However, other supplemental tests were requested to confirm the diagnosis and rule out other causes of liver disease. Abdominal ultrasound showed no liver or biliary tract changes. Serologic tests for hepatotropic viruses were negative, as were tests for hepatic autoimmunity and other connective tissue diseases, iron profile, copper, and ceruloplasmin. Infectious, autoimmune, or metabolic causes were thus ruled out.
The pattern of dissociated cholestasis gradually improved upon drug discontinuation until complete normalization occurred 12 weeks later (Table 1), which supported the diagnosis of methimazole-induced acute cholestatic hepatitis.
Changes in laboratory parameters over time.
| Laboratory tests | Baseline (without MMZa) | 4 months after MMZ start | 1 month after MMZ discontinuation | 3 months after MMZ discontinuation | Currently |
| Total bilirubin(NR: 0.2–1.4mg/dL) | 0.5 | 0.4 | 0.6 | 0.8 | 0.6 |
| Alkaline phosphatase(NR: 38–126U/L) | 76 | 367 | 216 | 64 | 23 |
| GOT/AST(NR: 17–59U/L) | 36 | 73 | 37 | 28 | 24 |
| GPT/ALT(NR: 21–72U/L) | 48 | 200 | 84 | 49 | 52 |
| GGT(NR: 11–73U/L) | 21 | 1163 | 716 | 60 | 29 |
| LDH(NR: 313–618U/L) | 483 | 488 | 483 | 451 | 422 |
| TSH(NR: 0.55–4.78mcU/mL) | 0.009 | 0.005 | 0.015 | 0.035 | 3.71 |
| Free T4(NR: 0.89–1.76mg/dL) | 4.21 | 2.09 | 2.19 | 2.05 | 1.3 |
| Free T3(NR: 2.3–4.2pg/mL) | 6.59 | 3.31 | 3.89 | 3.75 | 2 |
Once the condition was resolved, total thyroidectomy was decided to achieve short or mid-term control of thyrotoxicosis in order to prevent the exacerbation of cardiac disease. Three months after thyroid resection, the patient was symptom-free and had thyroid hormone levels in the normal range on 100mcg daily of levothyroxine sodium.
DiscussionThis letter reports a new case of acute cholestatic hepatitis secondary to treatment with methimazole. Although this is an adverse effect with an incidence less than 1% and occurring more commonly with propylthiouracyl, it should be taken into account because of its high mortality rate, close to 20–25%.6
The typical presentation is a cholestatic syndrome characterized by jaundice, biliuria, acholia, and pruritus, together with an increase in cholestasis parameters, and it is frequently associated with moderate transaminase elevation. Its diagnosis is supported by the occurrence of the condition usually from two weeks to three months after the start of treatment with thioamides and by observation of the clinical picture. However, our patient had a pattern of dissociated cholestasis, which made a clinical diagnosis difficult.
As already discussed, this is an exclusionary diagnosis. The only way to confirm the diagnosis is by histological examination, which in patients treated with methimazole or carbimazole would show hepatocanalicular cholestasis. By contrast, patients treated with propylthiouracyl usually have hepatocellular damage, which is more severe.5 Obviously, the collection of a histological sample is virtually impossible for practical and ethical reasons. On the other hand, the gradual normalization of hepatic parameters after drug discontinuation would indirectly support the diagnosis.
Thus, when the condition is clinically suspected, thioamide treatment should be discontinued and other causes of acute liver disease should be ruled out. However, it should be kept in mind that hyperthyroidism itself causes in up to 50% of patients increases in AP and GGT levels, which normalize after the recovery of a euthyroid state.5,6 However, such elevations are usually milder than when they are secondary to treatment with thioamides and are not associated with cholestatic patterns.
The most recent guidelines recommend that liver function abnormalities should be ruled out before anti-thyroid treatment is started, but not routinely during follow-up. They only recommend that a liver profile be requested when patients have symptoms and signs consistent with liver involvement, especially if they are receiving propylthiouracyl.1 In the case reported, an early diagnosis could not have been made because the patient had no clinical signs and was being treated with methamizole, with the resultant potential implications. It should be noted that the prevalence of liver parameter changes with the other two thioamides is so low that in a case such as the one reported, if the liver profile is not tested, this side effect may be overlooked. On the other hand, this has little practical relevance because the proportion of cases with asymptomatic elevations showing a poor course is probably very low.
Because of the frequency and severity of the consequences, the measurement of liver function parameters, either at regular intervals or for any clinical suspicion, is clearly needed in subjects treated with propylthiouracyl.
The question is whether routine liver profiles in patients treated with methimazole or carbimazole would be cost-effective since, as already noted, liver iatrogeny with such drugs is uncommon and results in cholestatic patterns which may be diagnosed clinically. The prevalence of liver abnormalities with these drugs is very likely to be higher, but they possibly run an indolent course and resolve spontaneously.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Francés Artigas C, et al. Colestasis disociada: una complicación infrecuente del tratamiento con tionamidas. Endocrinol Nutr. 2013;60:340–2.