The aim of this review was to assess the effectiveness to reduce clinical adverse events and safety of insulin administered in basal-bolus-corrector or basal-corrector regimens (BB) versus a sliding scale scheme (SS) in patients with diabetes or newly diagnosed hyperglycemia admitted to a conventional (not critical) medical or surgical hospital ward.
MethodA Medline search was conducted. The Odds ratio was the main summary measure. A random effects model with the Mantel–Haenszel procedure was used.
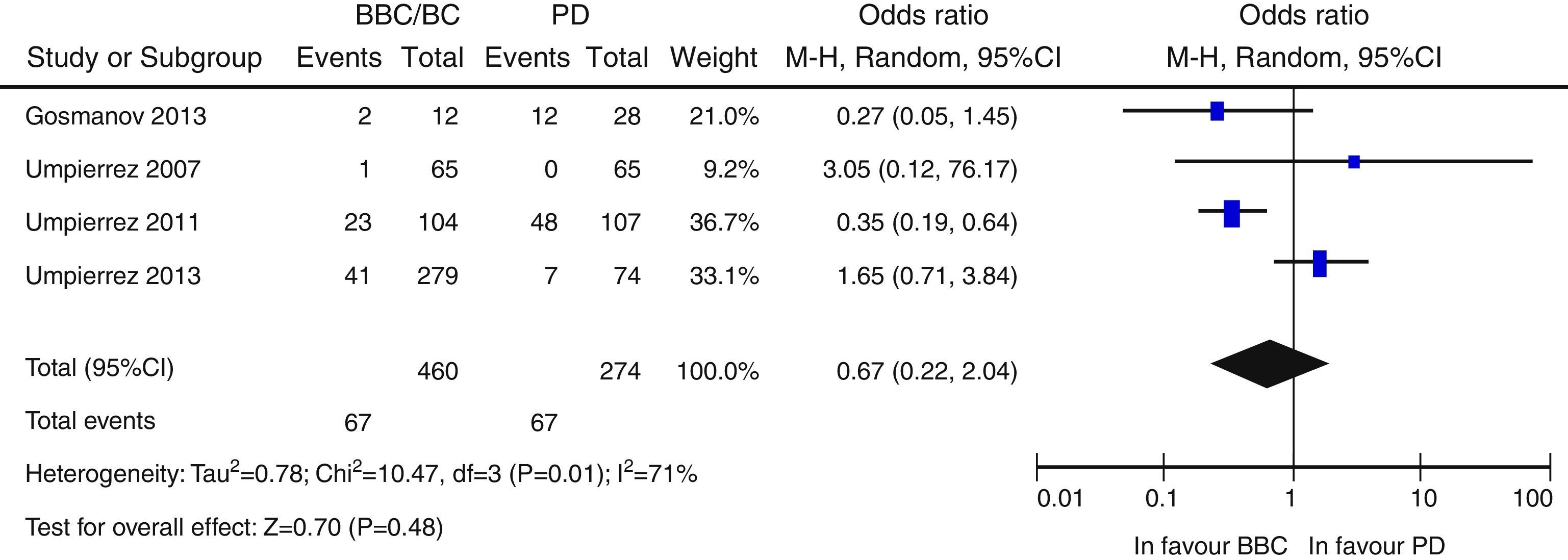
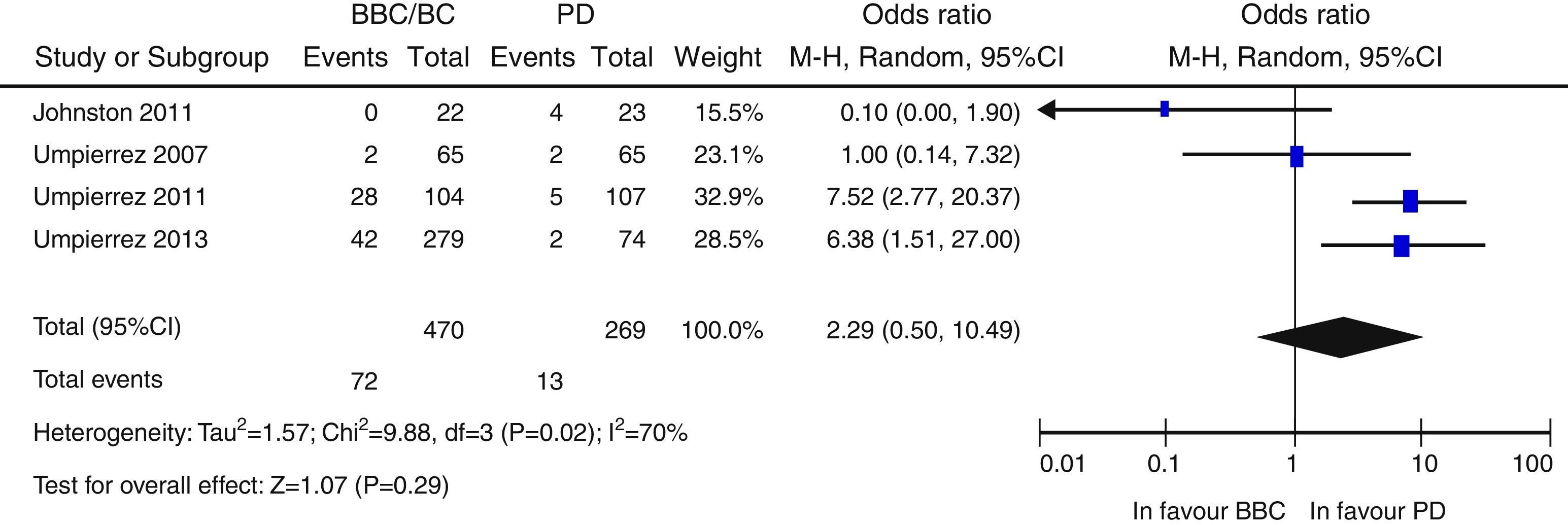
ResultsA total of 957 citations were collected, of which nine were finally included in the systematic review. Patients in the BB group had better blood glucose control than those with SS. Overall, there was a nonsignificant trend to a lower risk of adverse events in the BB as compared to the SS group (OR 0.67 [95% CI: 0.22–2.04], [I2=71%]). There was a nonsignificant trend to an increased risk of hypoglycemia in the BB group (OR 2.29 [95% CI: 0.50–10.49] [I2=70%]).
ConclusionDespite its benefit for glycemic control during hospitalization, this review did not show that use of the BB scheme decreases clinical events in patients hospitalized in a conventional ward. Because of heterogeneity of the results, we think that clinical trials are needed addressing its effect in patient subgroups in which the BB scheme may be used safely and with longer follow-up periods.
El objetivo de esta revisión es evaluar la efectividad para disminuir los eventos adversos clínicos y la seguridad de la insulinoterapia en régimen bolo-basal-corrector o basal-corrector frente a la insulinoterapia en «pauta deslizante», en pacientes con diabetes o con hiperglucemia de reciente diagnóstico ingresados en una planta de hospitalización convencional, no críticos, tanto médica como quirúrgica.
MétodoSe realizó búsqueda en Medline. La odds ratio fue la medida resumen principal. Se empleó un modelo de efectos aleatorios con la técnica de Mante-Haenszel.
ResultadosNovecientas cincuenta y siete citas de las cuales 9 fueron finalmente incluidas en la revisión sistemática. Los pacientes en el grupo BB tuvieron un mejor control glucémico que aquellos con PD. Globalmente, se objetiva una tendencia no significativa hacia un menor riesgo de eventos adversos en el grupo BB frente a PD (OR 0,67 —IC 95%: 0,22-2,04— [I2=71%]). Existe una tendencia no significativa hacia un mayor riesgo de hipoglucemia en el grupo BB (OR: 2,29; IC 95% 0,50-10,49 [I2=70%]).
ConclusiónA pesar de su beneficio para el control glucémico durante la hospitalización, esta revisión no ha objetivado que el uso de la pauta BB disminuya eventos clínicos en pacientes hospitalizados en planta convencional. Debido a la heterogeneidad en los resultados, consideramos que se requieren ensayos clínicos que contemplen su efecto en subgrupos de pacientes en los que la pauta BB se pueda usar de forma segura y con períodos de seguimiento más prolongados.
Diabetes mellitus (DM) is a disease with an increasing prevalence and a strong impact on health systems in most countries with stable economic development. In Spain, 14%1 of the population is estimated to have DM, accounting for 5809 million euros in direct costs and 17,650 million euros in indirect costs (including work absenteeism, sick leave, early retirement, and early mortality).2 Diabetes also causes a wide range of both acute and chronic complications, and its prevalence in the hospitalized population is therefore even greater. A cross-sectional study conducted in Spain found that up to 26% of patients hospitalized in both medical and surgical wards experience hyperglycemia during their hospital stay,3 and a rate up to 38% may be seen in data reported in the United States.4
Pathophysiologically, hyperglycemia leads to cell damage and causes immune dysfunction by several mechanisms (the release of pro-inflammatory cytokines, impaired neutrophil function, the release of oxygen free radicals, amongst others).5 Thus, the harmful effect of hyperglycemia during hospitalization has been established in various clinical settings in both critically ill and non-critically ill patients.4 Higher postoperative rates of complications (including infections) and mortality are seen in patients with poor glycemic control at admission,6,7 which is also a mortality predictor in patients admitted for myocardial infarction8 or stroke.9 Hyperglycemia is also related to greater infection rates in patients undergoing bone marrow transplant,10 and to shorter complete remissions and greater mortality in patients admitted for the treatment of acute lymphoblastic leukemia,11 among other examples.
Hyperglycemia occurring during hospital admission was traditionally controlled using the so-called “sliding scale insulin” (SSI) regimens, consisting of the administration of regular insulin only based on blood glucose control before meals. The value of such regimens, inconsistent with physiological insulin secretion, has been widely refuted.12 Observational studies have failed to demonstrate the efficacy of SSI for blood glucose control,13,14 and more recent clinical trials have shown its inferiority to so-called basal-bolus (BB) regimens.15,16 Despite the foregoing, because of its simplicity and, probably, because of the fear of hyperglycemia by clinicians, SSI continues to be widely used today. In our working environment, up to 65% of hospitalized patients with diabetes are treated with SSI only.3
This situation has prompted the conduct in the past decade of several studies intended to verify the effectiveness of other treatment regimens for blood glucose control during hospitalization. As previously noted, BB regimens, in which one part of the insulin is administered as lente or ultralente insulin to cover fasting hyperglycemia, have been shown to be effective for achieving adequate blood glucose control during the hospital stay.15–17 Less evidence exists, however, for the effect of BB regimens on the clinical prognosis of patients.18 To date, it has only been shown that the use of BB regimens decreases infection rates in surgical patients.16,19
The purpose of this review was to assess both the effectiveness in decreasing clinical adverse events and the safety of basal-bolus-corrective or basal-corrective insulin therapy as compared to “sliding scale” insulin therapy in patients with newly diagnosed diabetes or hyperglycemia admitted to a general medical or surgical hospital ward.
Patients and methodsThis review was conducted following the PRISMA guidelines,20,21 and the checklist is attached as supplemental material in Annex 1 (Table A1 of Annex 1).
Basal-bolus or basal-corrective (BB) regimens are defined as the administration to patients of fixed-dose lente or ultralente insulin (basal) together with boluses of ultra rapid-acting insulin associated with food intake (boluses may be fixed [prandial] and/or depend on blood glucose before intake [corrective]). “Sliding scale insulin” (SSI) regimens are defined as the administration of insulin boluses only based on capillary blood glucose monitoring before meals. An adverse event is defined as any complication recorded, including mortality, during the follow-up period defined in the different studies, as well as the number of hypoglycemic attacks.
A search was conducted in the Medline database, using PubMed, from its origin to 26 September 2014, and the search was repeated before data analysis (15 March 2015). References to relevant articles were also manually reviewed. The terms sliding scale, basal bolus, non-critically ill, inpatients, hospitalized patients, hyperglycemia and diabetes mellitus were used in the search. The complete search chain is included in Annex 1 (Table A2 of Annex 1).
Articles that met the following inclusion criteria were selected: clinical trials and cohort studies, both prospective and retrospective, including patients with newly-diagnosed hyperglycemia and/or a prior diagnosis of diabetes (irrespective of type) admitted to a general hospital ward. Intervention involved treatment using a BB regimen, and a control group given insulin on an SSI regimen. Finally, data on mortality and/or complications during hospital stay or follow-up (hospital-acquired infection, cardiovascular complications, mean length of stay) and/or safety data (hypoglycemia) were required. The exclusion criteria were as follows: patients under 18 years of age, admission to critical care units, intravenous insulin therapy and/or acute complications of diabetes (hyperosmolar hyperglycaemic state/diabetic ketoacidosis) at admission. Studies published in languages other than English or Spanish were also excluded. A single author was responsible for the final review and the selection of articles.
The Cochrane Collaboration's tool for assessing risk of bias in clinical trials was used.22 The Newcastle–Ottawa scale was used to assess the quality of the cohort studies.23 No a priori limit was established for the quality of articles to be included in the review or subsequent meta-analysis.
The following variables were collected from the selected articles using a predesigned form: author, year of publication, study location and period, design, age and sex, prior diabetes and its type, stress hyperglycemia, reason for admission, admission to medical or surgical ward, use of corticosteroids, type and dose of corticosteroids, diabetes treatment before admission, study exclusion criteria, HbA1c, use of a BB regimen in the intervention arm, use of an SSI regimen in the control group, type and dose of insulins used in both groups and regimen type and adjustments, number of injections of rapid-acting insulin in the SSI group, use of oral antidiabetic drugs during hospitalization, blood glucose control goal in the study, primary and secondary study endpoints, adverse event definition, blood glucose control, number of adverse events, mortality, and hypoglycemia in each group, and follow-up period.
The odds ratio (OR) was used as the main effect measure for each objective. The effectiveness of the intervention was assessed using a composite endpoint that included all clinically significant adverse events recorded in the studies, including mortality. Safety was assessed based on the number of hypoglycaemic attacks recorded. The Mantel–Haenszel (MH) procedure with a random effects model was used. Results are shown in a forest plot. Heterogeneity was analyzed using I statistics.2 A separate sensitivity analysis of the results of the clinical trials was planned. Publication bias was analyzed using funnel plots.
Microsoft Excel (2010) software was used for article selection, risk of bias assessment, and data collection. Literature references were managed using Mendeley Desktop software (version 1.13.8), and data analysis was performed using RevMan software (Review Manager. version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012).
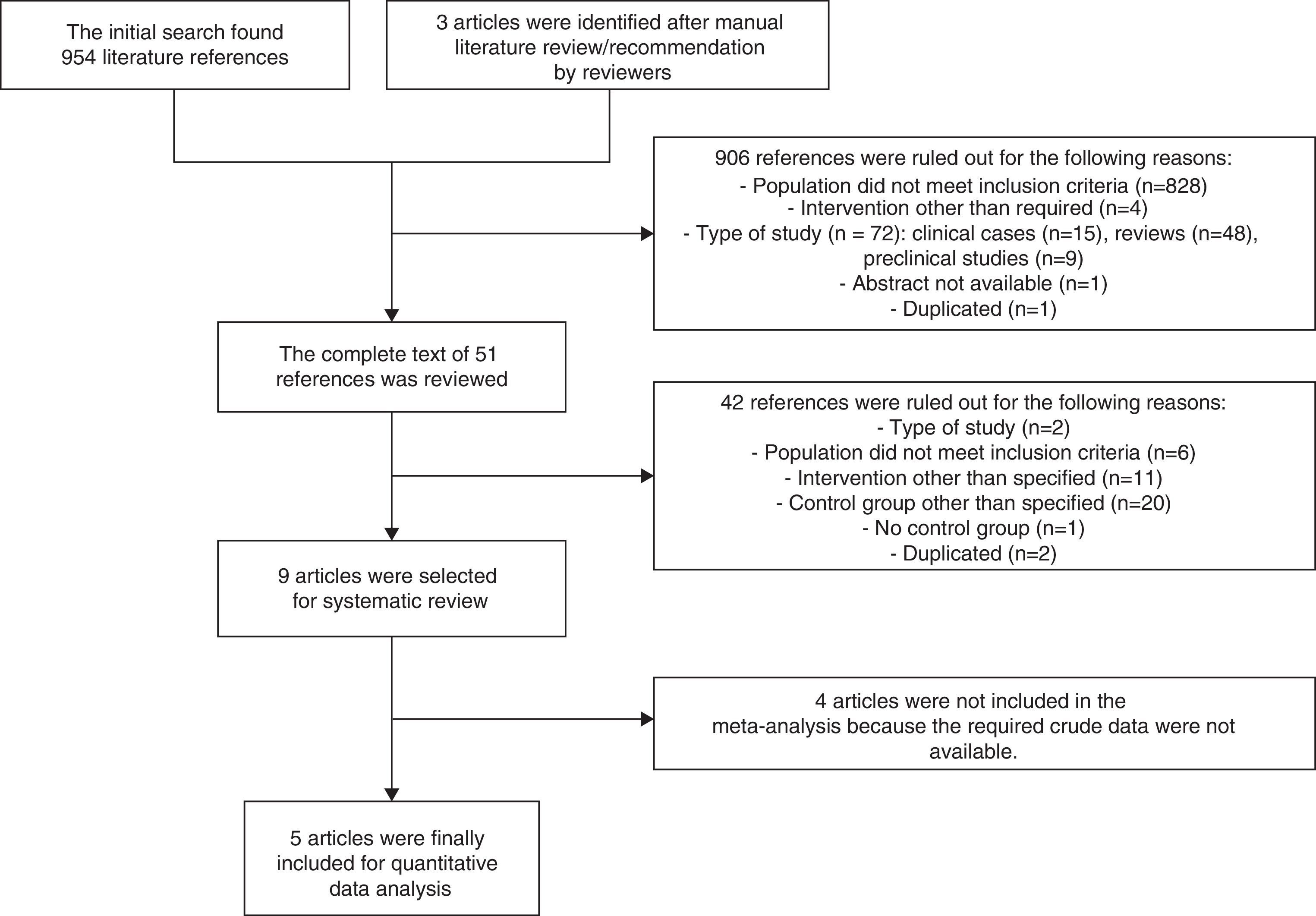
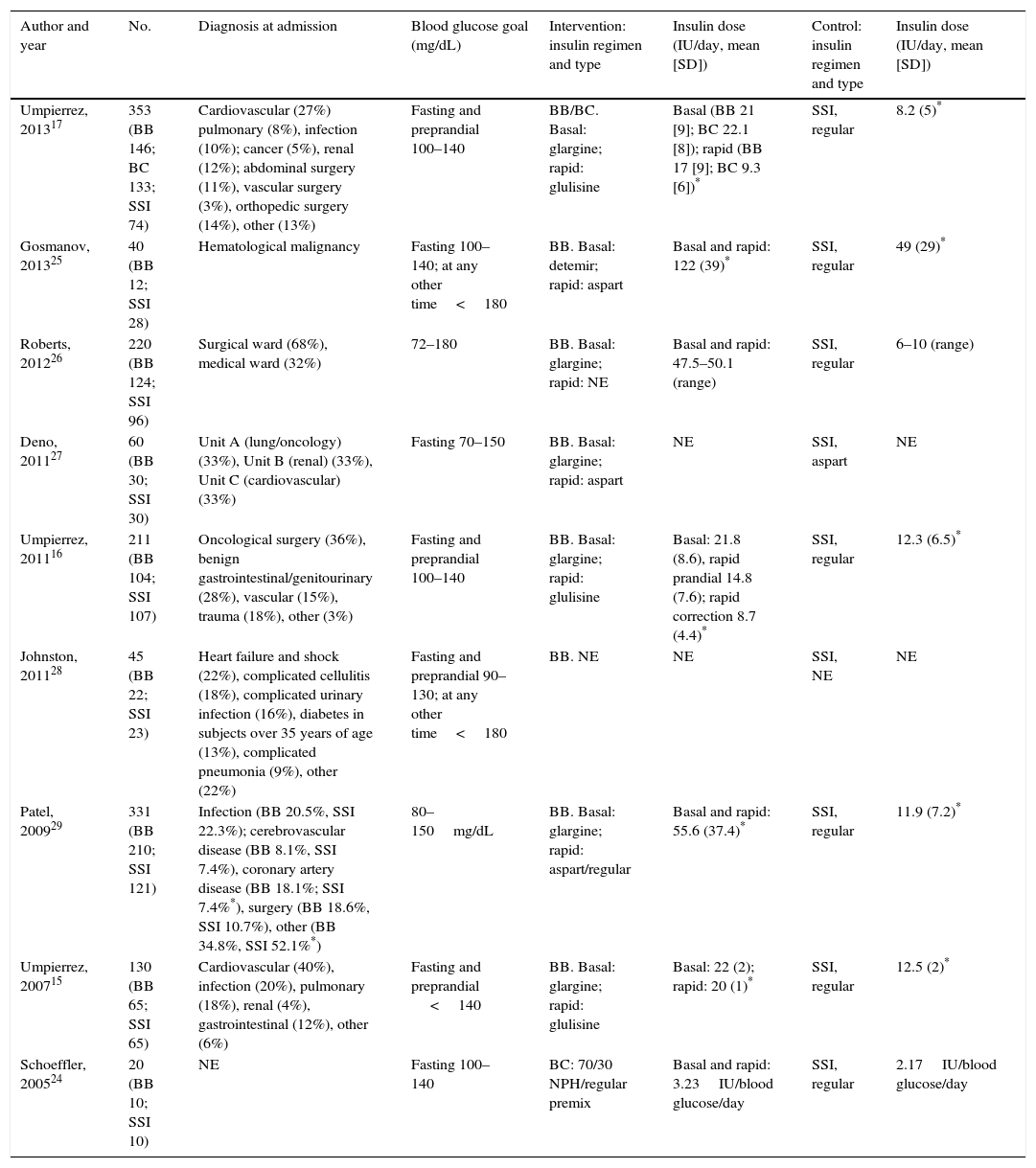
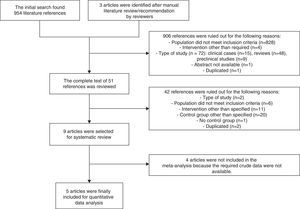
ResultsA total of 957 references were identified, of which 9 (4 clinical trials15–17,24 and 5 retrospective cohort studies25–29) finally met the criteria for inclusion in the systematic review. Studies by Roberts et al.,26 Deno et al.,27 Schoeffler et al.,24 and Patel et al.29 were finally not included in the meta-analysis because the crude data required were not accessible (Fig. 1).
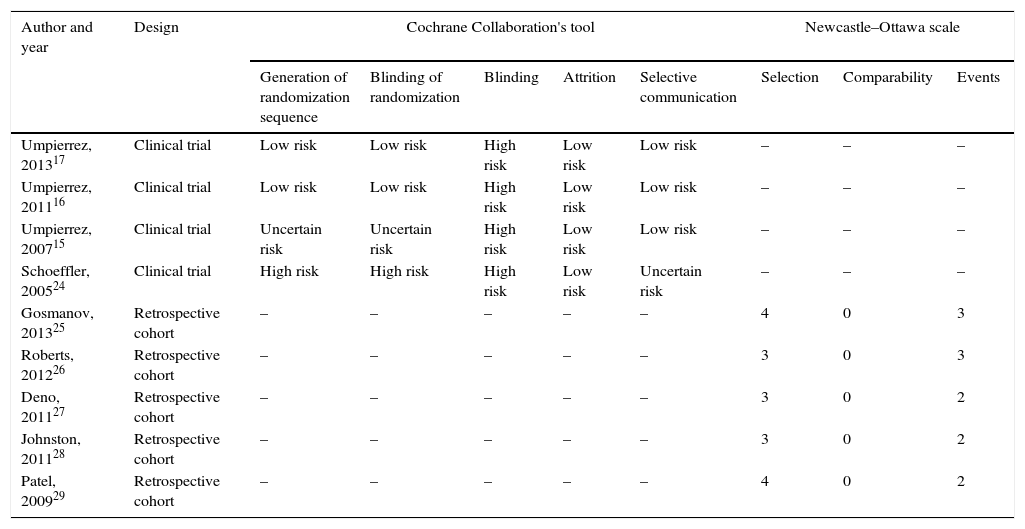
Table 1 summarizes the assessment of risk of bias in the studies included. Table 2 details the study characteristics. All the studies were conducted in the United States, except for the Roberts et al. study26 (Australia). They were all of patients with a prior diagnosis of type 2 DM with the exception of the studies conducted by Deno et al.,27 which included patients with stress hyperglycemia, and by Patel et al.,29 which enrolled patients with type 1 DM and stress hyperglycemia, although most patients in this study had a prior diagnosis of type 2 DM. The proportions of males enrolled into the different studies ranged from 32% to 72%, and mean age ranged from 55.6 (SD 7.3) to 75.7 (SD 10) years. The use of steroids was an exclusion criterion in all studies, except for the Gosmanov et al. study,25 where the use of dexamethasone was an inclusion criterion. Finally, the population in the studies included had a wide variety of diagnoses upon admission (Table 2). All groups, except for Schoeffler et al.24 (who used a NPH/regular mixture) and Johnston et al.28 (who did not report the regimen used) used as basal a ultralente (glargine15–17,26,27 or detemir25) insulin, and as prandial/corrective ultra rapid-acting (aspart or glulisine) or regular insulin.29 In the control group, regular insulin was used in most studies.15–17,24–26,29 The daily insulin doses used were consistently higher in the BB as compared to the SSI group in all the studies considered.15–17,24–26,29 Oral antidiabetic drugs were discontinued during the hospital stay in the clinical trials,15–17 but were allowed in most observational studies.24,26,28
Assessment of risk of bias in the articles included.
| Author and year | Design | Cochrane Collaboration's tool | Newcastle–Ottawa scale | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Generation of randomization sequence | Blinding of randomization | Blinding | Attrition | Selective communication | Selection | Comparability | Events | ||
| Umpierrez, 201317 | Clinical trial | Low risk | Low risk | High risk | Low risk | Low risk | – | – | – |
| Umpierrez, 201116 | Clinical trial | Low risk | Low risk | High risk | Low risk | Low risk | – | – | – |
| Umpierrez, 200715 | Clinical trial | Uncertain risk | Uncertain risk | High risk | Low risk | Low risk | – | – | – |
| Schoeffler, 200524 | Clinical trial | High risk | High risk | High risk | Low risk | Uncertain risk | – | – | – |
| Gosmanov, 201325 | Retrospective cohort | – | – | – | – | – | 4 | 0 | 3 |
| Roberts, 201226 | Retrospective cohort | – | – | – | – | – | 3 | 0 | 3 |
| Deno, 201127 | Retrospective cohort | – | – | – | – | – | 3 | 0 | 2 |
| Johnston, 201128 | Retrospective cohort | – | – | – | – | – | 3 | 0 | 2 |
| Patel, 200929 | Retrospective cohort | – | – | – | – | – | 4 | 0 | 2 |
Characteristics of the studies included: description of population and intervention.
| Author and year | No. | Diagnosis at admission | Blood glucose goal (mg/dL) | Intervention: insulin regimen and type | Insulin dose (IU/day, mean [SD]) | Control: insulin regimen and type | Insulin dose (IU/day, mean [SD]) |
|---|---|---|---|---|---|---|---|
| Umpierrez, 201317 | 353 (BB 146; BC 133; SSI 74) | Cardiovascular (27%) pulmonary (8%), infection (10%); cancer (5%), renal (12%); abdominal surgery (11%), vascular surgery (3%), orthopedic surgery (14%), other (13%) | Fasting and preprandial 100–140 | BB/BC. Basal: glargine; rapid: glulisine | Basal (BB 21 [9]; BC 22.1 [8]); rapid (BB 17 [9]; BC 9.3 [6])* | SSI, regular | 8.2 (5)* |
| Gosmanov, 201325 | 40 (BB 12; SSI 28) | Hematological malignancy | Fasting 100–140; at any other time<180 | BB. Basal: detemir; rapid: aspart | Basal and rapid: 122 (39)* | SSI, regular | 49 (29)* |
| Roberts, 201226 | 220 (BB 124; SSI 96) | Surgical ward (68%), medical ward (32%) | 72–180 | BB. Basal: glargine; rapid: NE | Basal and rapid: 47.5–50.1 (range) | SSI, regular | 6–10 (range) |
| Deno, 201127 | 60 (BB 30; SSI 30) | Unit A (lung/oncology) (33%), Unit B (renal) (33%), Unit C (cardiovascular) (33%) | Fasting 70–150 | BB. Basal: glargine; rapid: aspart | NE | SSI, aspart | NE |
| Umpierrez, 201116 | 211 (BB 104; SSI 107) | Oncological surgery (36%), benign gastrointestinal/genitourinary (28%), vascular (15%), trauma (18%), other (3%) | Fasting and preprandial 100–140 | BB. Basal: glargine; rapid: glulisine | Basal: 21.8 (8.6), rapid prandial 14.8 (7.6); rapid correction 8.7 (4.4)* | SSI, regular | 12.3 (6.5)* |
| Johnston, 201128 | 45 (BB 22; SSI 23) | Heart failure and shock (22%), complicated cellulitis (18%), complicated urinary infection (16%), diabetes in subjects over 35 years of age (13%), complicated pneumonia (9%), other (22%) | Fasting and preprandial 90–130; at any other time<180 | BB. NE | NE | SSI, NE | NE |
| Patel, 200929 | 331 (BB 210; SSI 121) | Infection (BB 20.5%, SSI 22.3%); cerebrovascular disease (BB 8.1%, SSI 7.4%), coronary artery disease (BB 18.1%; SSI 7.4%*), surgery (BB 18.6%, SSI 10.7%), other (BB 34.8%, SSI 52.1%*) | 80–150mg/dL | BB. Basal: glargine; rapid: aspart/regular | Basal and rapid: 55.6 (37.4)* | SSI, regular | 11.9 (7.2)* |
| Umpierrez, 200715 | 130 (BB 65; SSI 65) | Cardiovascular (40%), infection (20%), pulmonary (18%), renal (4%), gastrointestinal (12%), other (6%) | Fasting and preprandial <140 | BB. Basal: glargine; rapid: glulisine | Basal: 22 (2); rapid: 20 (1)* | SSI, regular | 12.5 (2)* |
| Schoeffler, 200524 | 20 (BB 10; SSI 10) | NE | Fasting 100–140 | BC: 70/30 NPH/regular premix | Basal and rapid: 3.23IU/blood glucose/day | SSI, regular | 2.17IU/blood glucose/day |
BB, basal/bolus-correction; BC, basal-correction; SD, standard deviation; DM, diabetes mellitus; NE, not specified; SSI, sliding scale insulin regimen.
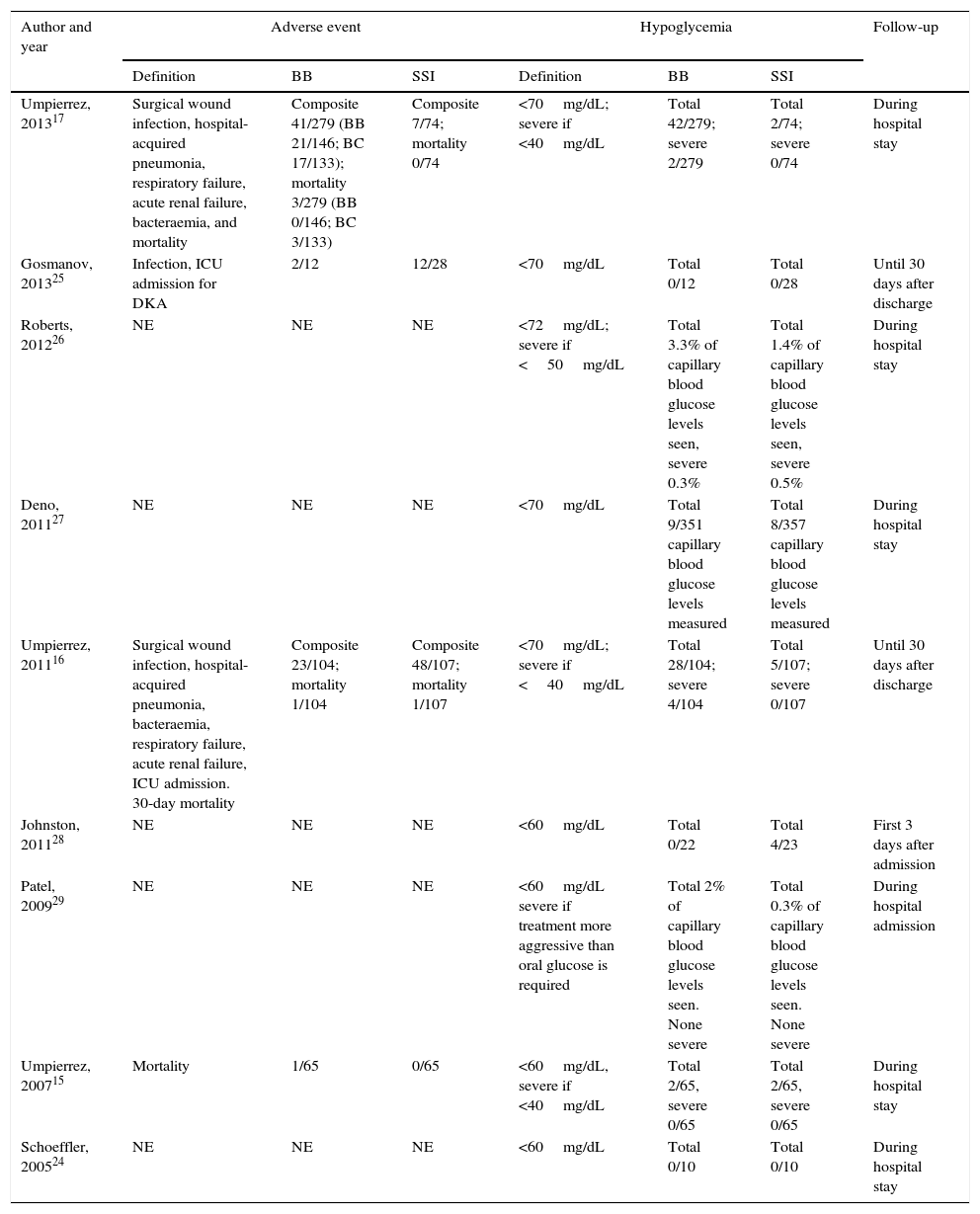
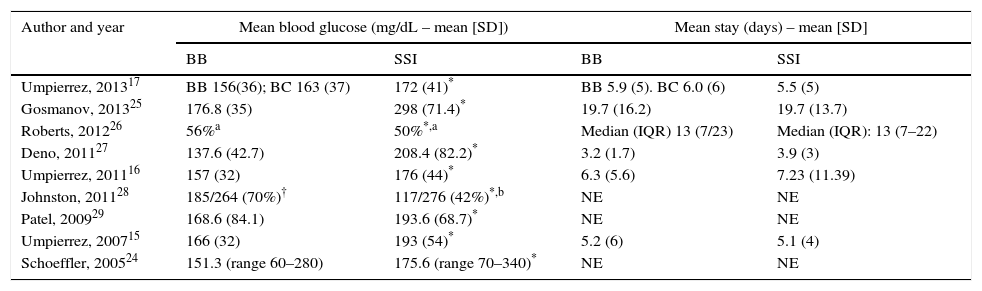
Table 3 summarizes the results of the studies included. No significant difference was found in mean hospital stay between the BB and SSI groups in any of the studies included. Except in the Johnston et al. study,28 patients in the BB group had better blood glucose control than those given insulin only in accordance with the SSI regimen (Table 4).
Results of the individual studies: adverse events and hypoglycemia.
| Author and year | Adverse event | Hypoglycemia | Follow-up | ||||
|---|---|---|---|---|---|---|---|
| Definition | BB | SSI | Definition | BB | SSI | ||
| Umpierrez, 201317 | Surgical wound infection, hospital-acquired pneumonia, respiratory failure, acute renal failure, bacteraemia, and mortality | Composite 41/279 (BB 21/146; BC 17/133); mortality 3/279 (BB 0/146; BC 3/133) | Composite 7/74; mortality 0/74 | <70mg/dL; severe if <40mg/dL | Total 42/279; severe 2/279 | Total 2/74; severe 0/74 | During hospital stay |
| Gosmanov, 201325 | Infection, ICU admission for DKA | 2/12 | 12/28 | <70mg/dL | Total 0/12 | Total 0/28 | Until 30 days after discharge |
| Roberts, 201226 | NE | NE | NE | <72mg/dL; severe if <50mg/dL | Total 3.3% of capillary blood glucose levels seen, severe 0.3% | Total 1.4% of capillary blood glucose levels seen, severe 0.5% | During hospital stay |
| Deno, 201127 | NE | NE | NE | <70mg/dL | Total 9/351 capillary blood glucose levels measured | Total 8/357 capillary blood glucose levels measured | During hospital stay |
| Umpierrez, 201116 | Surgical wound infection, hospital-acquired pneumonia, bacteraemia, respiratory failure, acute renal failure, ICU admission. 30-day mortality | Composite 23/104; mortality 1/104 | Composite 48/107; mortality 1/107 | <70mg/dL; severe if <40mg/dL | Total 28/104; severe 4/104 | Total 5/107; severe 0/107 | Until 30 days after discharge |
| Johnston, 201128 | NE | NE | NE | <60mg/dL | Total 0/22 | Total 4/23 | First 3 days after admission |
| Patel, 200929 | NE | NE | NE | <60mg/dL severe if treatment more aggressive than oral glucose is required | Total 2% of capillary blood glucose levels seen. None severe | Total 0.3% of capillary blood glucose levels seen. None severe | During hospital admission |
| Umpierrez, 200715 | Mortality | 1/65 | 0/65 | <60mg/dL, severe if <40mg/dL | Total 2/65, severe 0/65 | Total 2/65, severe 0/65 | During hospital stay |
| Schoeffler, 200524 | NE | NE | NE | <60mg/dL | Total 0/10 | Total 0/10 | During hospital stay |
BB, basal/bolus-correction; BC, basal-correction; DKA, diabetic ketoacidosis; NE, not specified; SSI, sliding scale insulin; ICU, intensive care unit.
Results of the studies included: blood glucose control and mean hospital stay.
| Author and year | Mean blood glucose (mg/dL – mean [SD]) | Mean stay (days) – mean [SD] | ||
|---|---|---|---|---|
| BB | SSI | BB | SSI | |
| Umpierrez, 201317 | BB 156(36); BC 163 (37) | 172 (41)* | BB 5.9 (5). BC 6.0 (6) | 5.5 (5) |
| Gosmanov, 201325 | 176.8 (35) | 298 (71.4)* | 19.7 (16.2) | 19.7 (13.7) |
| Roberts, 201226 | 56%a | 50%*,a | Median (IQR) 13 (7/23) | Median (IQR): 13 (7–22) |
| Deno, 201127 | 137.6 (42.7) | 208.4 (82.2)* | 3.2 (1.7) | 3.9 (3) |
| Umpierrez, 201116 | 157 (32) | 176 (44)* | 6.3 (5.6) | 7.23 (11.39) |
| Johnston, 201128 | 185/264 (70%)† | 117/276 (42%)*,b | NE | NE |
| Patel, 200929 | 168.6 (84.1) | 193.6 (68.7)* | NE | NE |
| Umpierrez, 200715 | 166 (32) | 193 (54)* | 5.2 (6) | 5.1 (4) |
| Schoeffler, 200524 | 151.3 (range 60–280) | 175.6 (range 70–340)* | NE | NE |
BB, basal/bolus-correction; BC, basal-correction; SD, standard deviation; NE, not specific; SSI, sliding scale insulin; IQR, interquartile range.
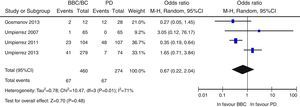
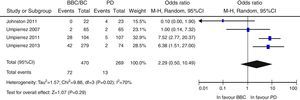
Overall, there was a non-significant trend to a lower risk of adverse events in the BB group as compared to the SSI group (OR 0.67 [95% CI: 0.22–2.04], I2=71%) (Fig. 2). A similar result was seen when the clinical trials were separately analyzed15–17 (OR 0.89 [95% CI: 0.23–3.50], I2=79%), and also when only infectious complications were analyzed (OR 0.5 [95% CI: 0.17–1.49], I2=54%).16,17,25 There was also a non-significant trend to a greater risk of hyperglycemia in the BB group (OR 2.29, 95% CI: 0.50–10.49 [I2=70%]) (Fig. 3), which was confirmed when the results of the clinical trials were separately analyzed15–17 (OR 4.72, 95% CI: 1.68–13.2, I2=38%). An exploratory analysis which was conducted into the risk of severe hyperglycemia in both groups found no significant differences between them (OR 3.73, 95% CI: 0.45–30.88, I2=0%), although this data was only provided by two studies.16,17
Finally, publication bias was assessed using funnel plots, which showed a significant asymmetry of the studies analyzing the risk of hypoglycemia. This suggests that there may be studies not included in our review. In fact, three of the studies selected24,26,27 could not be finally included in the analysis due to a lack of patient data.
DiscussionThe BB regimen has been shown to improve blood glucose control during hospitalization as compared to SSI. Thus, in the current review, glucose control was significantly better in the BB group in most of the studies included.15–17,24–27,29 Our review, however, could not show that improved blood glucose control derived from the use of a BB regimen decreases clinically significant adverse events as compared to the use of SSI. This result is similar to that reported in other reviews previously conducted.18,19 Thus, the Murad et al. review19 comparing the effect of intensive blood glucose control (using BB or intravenous insulin infusion) versus standard control in patients admitted to a general ward, found no reduction in mortality, the incidence of stroke, or acute myocardial infarction. There was however a lower infection rate in the intensive control group, mainly at the expense of studies conducted in surgical wards.
The first limitation of this study is that very few prospective studies have been conducted in general hospitalization settings with subcutaneous insulin. In fact, a previous review performed in 2011 by Kansaga et al.18 included no study conducted in general hospital wards, and the Murad et al. review19 only found 5 studies using subcutaneous insulin in BB regimens in general hospital wards. Six of the 9 studies included in our review16,17,25–28 were conducted over the past 5 years, which reflects the increasing concern for the problem of hyperglycemia in general hospital wards. However, most of the studies included come from the same research group,15–17 which may restrict the generalizability of the results.
On the other hand, adverse event incidence was not a primary objective in any of the studies included, except for the Umpierrez et al. study, 201116 (most events), so that the power of each individual study to detect differences may be inadequate.
An additional limitation of this study, shared with prior reviews,19 is the marked heterogeneity found in the analysis. In our view, this statistical heterogeneity reflects the significant differences in design between the studies included. First of all, the inclusion of both observational studies and clinical trials, as contemplated in the initial protocol, may represent a first source of heterogeneity. Furthermore, the definition of adverse events was not consistent in the studies included and, as noted above, only in the Umpierrez et al., 2011 study16 was the analysis of clinical events a primary objective. There were also marked differences in the length of follow-up, which may mean that some complications were underdiagnosed in studies with shorter follow-up (hospital-acquired infection, for instance, may occur up to 30 days after hospital discharge).
As regards hypoglycemia, the current review showed a trend to a greater risk of hyperglycemia in the BB group, which was confirmed in a separate analysis of the clinical trials. This finding is similar to the one reported by Murad et al.,19 who found a relative risk of hypoglycemia of 1.58 (95% CI: 0.97–2.57) in the intervention group. The negative effect of hypoglycemia in patients admitted to general hospital wards has been assessed in other studies.30,31 Thus, Turchin et al.30 found a relationship between hypoglycemia during the hospital stay and mortality in the following year. More recently, Kim et al.31 showed that hypoglycemia, after adjusting for other variables, doubled the risk of experiencing an adverse event (admission to an intensive care unit, hospital-acquired infection, or acute renal failure) in patients with diabetes during their stay at a hospital ward. Although the trend to hypoglycemia in the BB group may mask the benefits of intervention, we think that two qualifications to the results are needed. First, although hypoglycemia was associated in some studies with increased adverse events, other studies32,33 suggest that this harmful effect may be attributed to spontaneous hypoglycemia, but not to hypoglycaemic episodes occurring during the treatment of hyperglycemia. On the other hand, the Boucai et al. study33 demonstrated that the association of spontaneous hypoglycemia with mortality disappears after adjusting for comorbidity. The authors argue that the burden of this comorbidity, rather than hypoglycemia itself, is responsible for excess mortality. Based on the foregoing and because of the uncertainty about the role of hypoglycemia, some authors34 recommend the use of strategies to prevent hypoglycemia, together with moderate blood glucose control, in those patients more susceptible to the negative effect of hypoglycemia, such as the population with cardiovascular disease. The representation of patients with cardiovascular disease in the studies included in this review was variable, which may represent another determinant of the results achieved. Thus, the Umpierrez et al., 201116 and the Gosmanov et al. studies25 were the only ones that did not include patients with cardiovascular disease as a reason for admission, and both showed a decreased risk of events in the BB group (although statistical significance was not reached in the Gosmanov et al. study25).
Despite the above limitations, our study is the first to assess the clinical benefit of the BB regimen in hospitalized patients, and the intervention (insulin type and dosage) was similar in the various studies.
In conclusion, despite the benefits of the BB regimen for blood glucose control during hospitalization, this review has not shown that its use decreases clinical events in patients admitted to a general ward. However, these results should be analyzed with caution. Because of the heterogeneity of the results, we think that clinical trials are needed which address the effect of the BB regimen on patient subgroups in which the regimen may be used safely and with longer follow-up periods, in order to establish the actual benefit of the BB regimen during the hospital stay.
Conflicts of interestThe authors state that they have no conflicts of interest.
We thank the staff of the library of Hospital Universitario 12 de Octubre.
PRISMA guidelines.
| Section | Item | Verification | Recorded on page |
|---|---|---|---|
| Title | |||
| Title | 1 | Identification of the article as a systematic review, meta-analysis or both | 1 |
| Abstract | |||
| Structured summary | 2 | A structured summary including, where applicable, introduction, objectives, data source, study eligibility criteria, participants, interventions, method, results, limitations, conclusions, and applicability of key findings, registry number of systematic review | 13–14 |
| Introduction | |||
| Rationale | 3 | Describe the reason for performing the review in the context of what is already known | 2 |
| Objective | 4 | Explicitly discuss the research question detailing participants, interventions, comparison group, events, and study design (PICOS) | 3–4 |
| Methods | |||
| Protocol and registration | 5 | State if a review protocol is available, and if it may be accessed (e.g. website) and, if available, information about protocol registration | 5–7 |
| Eligibility criteria | 6 | Provide a detailed rationale for study characteristics (PICOS, follow-up time etc.) and article characteristics (date of publication, language, publication status) used as eligibility criteria | 5–7 |
| Information sources | 7 | Describe all information sources (databases, including dates, whether article authors have been contacted) included in the search and the date of the last search | 5–7 |
| Search | 8 | Show the electronic search strategy for at least one database, including the limits used, so that it may be repeated | 5–7 |
| Study selection | 9 | State the process for study selection (screening, selection for systematic review and meta-analysis if applicable) | 5–7 |
| Data collection | 10 | Describe the method used to collect data (use of an independently verified form in duplicate) and any process for obtaining and confirming data used by the researchers | 5–7 |
| Data items | 11 | List and define all variables reviewed in the data and any assumption or simplification made | 5–7 |
| Risk of bias in individual studies | 12 | Describe the methods used to assess risk of bias in individual studies and how this information was used for data synthesis | 5–7 |
| Summary measures | 13 | State the main summary measure | 5–7 |
| Synthesis of results | 14 | Describe the methods used to combine the results of the different studies and whether consistency measures have been performed (e.g. I2) for each meta-analysis | 5–7 |
| Risk of bias across studies | 15 | Specify the measures of risk of bias that may affect the cumulative evidence (e.g. publication bias, communication bias within studies) | 5–7 |
| Additional analyses | 16 | Describe the methods for any additional analysis (subgroups, sensitivity analysis, meta-regression etc.) and whether it was prespecified | 5–7 |
| Results | |||
| Study selection | 17 | Provide the number of studies screened, selected for detailed review, and included in the review, with the reasons for exclusion in each phase, ideally using a flow chart | 8, Fig. 1 |
| Study characteristics | 18 | Show the data taken from each study and the corresponding references | Table 2 |
| Risk of bias within studies | 19 | Report assessment of risk of bias in each study and, if available, its impact at event level | Table 1 |
| Results of individual studies | 20 | For all objectives considered (beneficial or harmful), report for each study (a) data summary for each intervention group and (b) estimated effect with confidence intervals, ideally using a forest plot | Tables 3 and 4, Figs. 2 and 3 |
| Risk of publication bias | 22 | Show in the results any assessment of publication bias | 9 |
| Additional analyses | 23 | Provide the results of any additional analysis performed (sensitivity, subgroups, meta-regression) | 9 |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings for each primary objective, consider their relevance | 10–11 |
| Limitations | 25 | Discuss limitations of studies at objective and review levels | 10–11 |
| Conclusions | 26 | General interpretation of results as a function of evidence and implications for future studies | 10–11 |
Complete search chain.
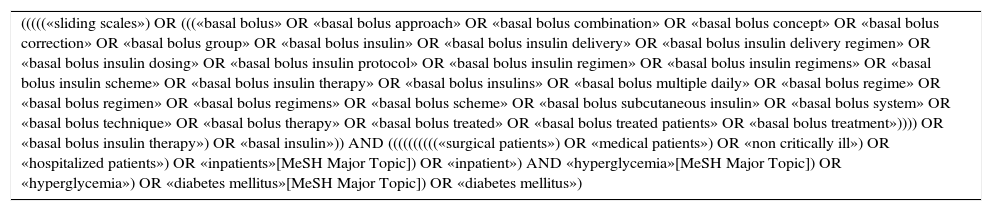
| (((((«sliding scales») OR (((«basal bolus» OR «basal bolus approach» OR «basal bolus combination» OR «basal bolus concept» OR «basal bolus correction» OR «basal bolus group» OR «basal bolus insulin» OR «basal bolus insulin delivery» OR «basal bolus insulin delivery regimen» OR «basal bolus insulin dosing» OR «basal bolus insulin protocol» OR «basal bolus insulin regimen» OR «basal bolus insulin regimens» OR «basal bolus insulin scheme» OR «basal bolus insulin therapy» OR «basal bolus insulins» OR «basal bolus multiple daily» OR «basal bolus regime» OR «basal bolus regimen» OR «basal bolus regimens» OR «basal bolus scheme» OR «basal bolus subcutaneous insulin» OR «basal bolus system» OR «basal bolus technique» OR «basal bolus therapy» OR «basal bolus treated» OR «basal bolus treated patients» OR «basal bolus treatment»)))) OR «basal bolus insulin therapy») OR «basal insulin»)) AND ((((((((((«surgical patients») OR «medical patients») OR «non critically ill») OR «hospitalized patients») OR «inpatients»[MeSH Major Topic]) OR «inpatient») AND «hyperglycemia»[MeSH Major Topic]) OR «hyperglycemia») OR «diabetes mellitus»[MeSH Major Topic]) OR «diabetes mellitus») |
Please cite this article as: Gómez Cuervo C, Sánchez Morla A, Pérez-Jacoiste Asín MA, Bisbal Pardo O, Pérez Ordoño L, Vila Santos J. Eficacia en la reducción de eventos adversos de la insulinoterapia en pauta bolo-basal frente a la pauta deslizante en pacientes con diabetes durante la hospitalización convencional: revisión sistemática de la literatura y metaanálisis. Endocrinol Nutr. 2016;63:145–156.