To analyze the efficacy and safety of empagliflozin combined with other oral hypoglycemic agents in patients with type 2 diabetes mellitus.
MethodsPooled analysis of three phase III trials in patients with type 2 diabetes mellitus (n=1801) who received placebo or empagliflozin 10 or 25mg once daily for 24 weeks, in combination with metformin, metformin+sulphonylurea or pioglitazone±metformin.
ResultsEmpagliflozin significantly decreased HbA1c (adjusted mean reduction vs placebo with empagliflozin 10mg: −0.58% [95% CI: −0.66; −0.49]; p<0.0001, and with empagliflozin 25mg: −0.62% [95% CI: −0.70; −0.53], p<0.0001), weight (adjusted mean reduction vs placebo with empagliflozin 10mg: −1.77kg [95% CI: −2.05; −1.48]; p<0.0001, and with empagliflozin 25mg: −1.96kg [95% CI: −2.24; −1.67], p<0.0001), and systolic and diastolic blood pressure (SBP/DBP). Adverse effect rates were 64% with placebo, 63.9% with empagliflozin 10mg, and 60.9% with empagliflozin 25mg. Documented episodes of hypoglycemia (≤70mg/dL and/or requiring care) occurred in 3.9% of patients with placebo, 6.9% of patients with empagliflozin 10mg, and 5.3% of patients with empagliflozin 25mg. Urinary tract infections developed in 9.4% of patients with placebo, 10.2% of patients with empagliflozin 10mg, and 8.3% of patients with empagliflozin 25mg. Genital infections were reported in 1.0% of patients with placebo, 4.6% of patients with empagliflozin 10mg, and 3.5% of patients with empagliflozin 25mg.
ConclusionsEmpagliflozin combined with other oral treatments decreased HbA1c, body weight, and SBP/DBP as compared to placebo, with a good safety and tolerability profile.
Analizar la eficacia y la seguridad de empagliflozina en combinación con otros hipoglucemiantes orales en pacientes con diabetes mellitus tipo 2.
MétodosAnálisis de 3 ensayos fase iii en pacientes con diabetes mellitus tipo 2 (n=1.801) que recibieron placebo, empagliflozina 10 o 25mg, una vez al día, durante 24 semanas, en combinación con metformina, metformina+sulfonilurea o pioglitazona±metformina.
ResultadosEmpagliflozina redujo significativamente la HbA1c (reducción media ajustada vs placebo con empagliflozina 10mg: –0.58% [IC 95%: –0,66; –0,49]; p<0,0001 y con empagliflozina 25mg –0,62% [IC 95%: –0,70; –0,53], p<0,0001), el peso (reducción media ajustada vs placebo con empagliflozina 10mg: –1,77kg [IC 95%: –2,05; –1,48]; p<0,0001 y con empagliflozina 25mg: –1,96kg [IC 95%: –2,24; –1,67], p<0,0001) y la presión arterial sistólica y diastólica. La frecuencia de efectos adversos fue del 64% con placebo, del 63,9% con empagliflozina 10mg y del 60,9% con empagliflozina 25mg. Las hipoglucemias confirmadas (≤70mg/dl y/o requiriendo asistencia) ocurrieron en un 3,9% de los pacientes con placebo, un 6,9% con empagliflozina 10mg y un 5,3% con empagliflozina 25mg. Las infecciones del tracto urinario acontecieron en un 9,4% con placebo, un 10,2% con empagliflozina 10mg y un 8,3% con empagliflozina 25mg. Las infecciones genitales se comunicaron en un 1,0% de los pacientes con placebo, un 4,6% con empagliflozina 10mg y un 3,5% con empagliflozina 25mg.
ConclusionesEmpagliflozina en combinación con otros tratamientos orales vs placebo disminuyó significativamente la HbA1c, el peso corporal y la presión arterial sistólica/diastólica, con un buen perfil de seguridad y tolerancia.
Metformin is the first choice treatment to achieve adequate blood glucose control in patients with type 2 diabetes mellitus (T2DM), combined with nutritional therapy and physical activity.1 However, this treatment becomes inadequate over time due to gradual impairment of insulin secretion by pancreatic β cells. The UKPDS study showed that therapeutic goals were not achieved in 40–50% of patients after two years of treatment with metformin,2,3 and in 70% at three years.4 Use of two or even three drugs when treatment goals are not achieved or maintained with metformin is currently recommended.1 While pharmacological options of similar efficacy are available, these have some limitations, including risk of hypoglycemia, weight increase, gastrointestinal effects, etc., and/or specific contraindications.5 Moreover, agents that stimulate insulin secretion lose efficacy when secretion is deficient due to loss of pancreatic β-cell function with disease progression.6,7 There is therefore a need for developing agents that effectively decrease hyperglycemia through new mechanisms of action independent from insulin secretion and which are not associated per se to weight increase or risk of hypoglycemia. These are some of the factors that prevent achievement or maintenance of blood glucose control goals in a significant proportion of patients with T2DM.8
Sodium-glucose co-transporter type 2 (SGLT2) inhibitors are a new family of hypoglycemic agents for the treatment of T2DM. Their mechanism of action is inhibition of glucose reabsorption in the kidney, promoting urinary excretion of glucose, regardless of residual insulin secretion. These drugs have a low risk of hypoglycemia and are associated to decreases in body weight and blood pressure.9
Empagliflozin is a highly selective SGLT2 inhibitor10 which has been shown to be effective for reducing HbA1c levels by decreasing fasting and postprandial plasma glucose, and to cause significant decreases in body weight and systolic (SBP) and diastolic (DBP) blood pressure. Empagliflozin is effective both as monotherapy and combined with other glucose-lowering drugs, including insulin.9,11–13
The purpose of this post hoc analysis was to assess the efficacy and safety of empagliflozin, combined with other oral agents, in patients with T2DM and inadequate blood glucose control as monotherapy or dual therapy.
Patients and methodsDesignA post hoc analysis of three Phase III multicenter, randomized, double-blind, placebo-controlled clinical trials.9,12,13 All three trials compared in patients with T2DM the efficacy and safety of empagliflozin (10mg/day or 25mg/day) vs placebo as combined therapy during 24 weeks. Study design and methods were reported in the original manuscripts.9,12,13 Before recruitment into the trials, patients received metformin,12 metformin+sulfonylurea,13 or pioglitazone±metformin9 for at least 12 weeks before randomization. Patients continued baseline treatment and were randomized to single daily doses of placebo, empagliflozin 10mg, or empagliflozin 25mg.
All clinical trials were conducted in compliance with the ethical principles of the Declaration of Helsinki and were consistent with good clinical practice and with the relevant regulatory requirements. They were all approved by the corresponding regulatory authorities and by independent ethics committees. Patients recruited were given adequate information, asked any questions they had, and signed the informed consent form before participating in the trials.
Study populationAll patients included in this analysis met all inclusion criteria and no exclusion criterion, as detailed in each clinical trial.9,12,13 Patients aged ≥18 years with uncontrolled T2DM (HbA1c 7–10%) despite diet, physical exercise, and a stable glucose-lowering treatment for more than 12 weeks, and with a BMI≤45kg/m2 were recruited. The main exclusion criteria were uncontrolled hyperglycemia (fasting blood glucose>240mg/dL, confirmed by a second measurement), acute coronary syndrome, stroke or transient ischemic attack in the three months prior to consent, and kidney or liver failure, amongst others.
Study variablesThe primary variable was in all trials the change seen in HbA1c levels from baseline to week 24 of treatment. Changes in body weight and SBP and DBP from study start to 24 weeks of treatment were assessed. The proportion of patients who achieved HbA1c levels lower than 7% after 24 weeks of treatment was also assessed.
The safety and tolerability of empagliflozin was analyzed based on the adverse effects (AEs) reported during the study and for up to 7 days after the last dose (according to the Medical Dictionary for Drug Regulatory Activities version 15.0) Results of several laboratory tests. electrocardiograms, and vital signs were also recorded. The incidence rate of some AEs of interest such as confirmed hypoglycemia (plasma glucose ≤70mg/dL and/or requiring assistance), urinary tract infection (UTI), and genital infection was also assessed.
Statistical analysisStatistical analysis of efficacy included randomized patients who had received one or more doses of study medication and had a baseline HbA1c measurement (complete analysis set). The primary analysis of efficacy, defined as change in HbA1c levels after 24 weeks of treatment was performed using an analysis of covariance, using baseline HbA1c as linear covariate. Baseline glomerular filtration rate estimated using the equation Modification of Diet in Renal Disease, geographical region, and treatment were considered fixed effects in the model. The same model was used for all other variables, considering the baseline value of each variable as additional linear covariate. In the comparison of groups treated with empagliflozin (10 and 25mg) to the placebo group, 95% confidence intervals and p-values were calculated, except in the comparison for the proportion of patients with baseline HbA1c levels ≥7.0% who achieved an HbA1c level <7.0% at 24 weeks of treatment, for which a descriptive analysis was considered. Patients who did not complete the study were considered treatment failures.
The safety analysis included all patients taking at least one dose of study medication (treated population), and results were given as absolute values and occurrence rate.
ResultsPatient characteristicsA total of 1081 patients were included in the post hoc analysis (596 with placebo, 606 with empagliflozin 10mg, and 599 with empagliflozin 25mg). Of these 1656 completed the study.
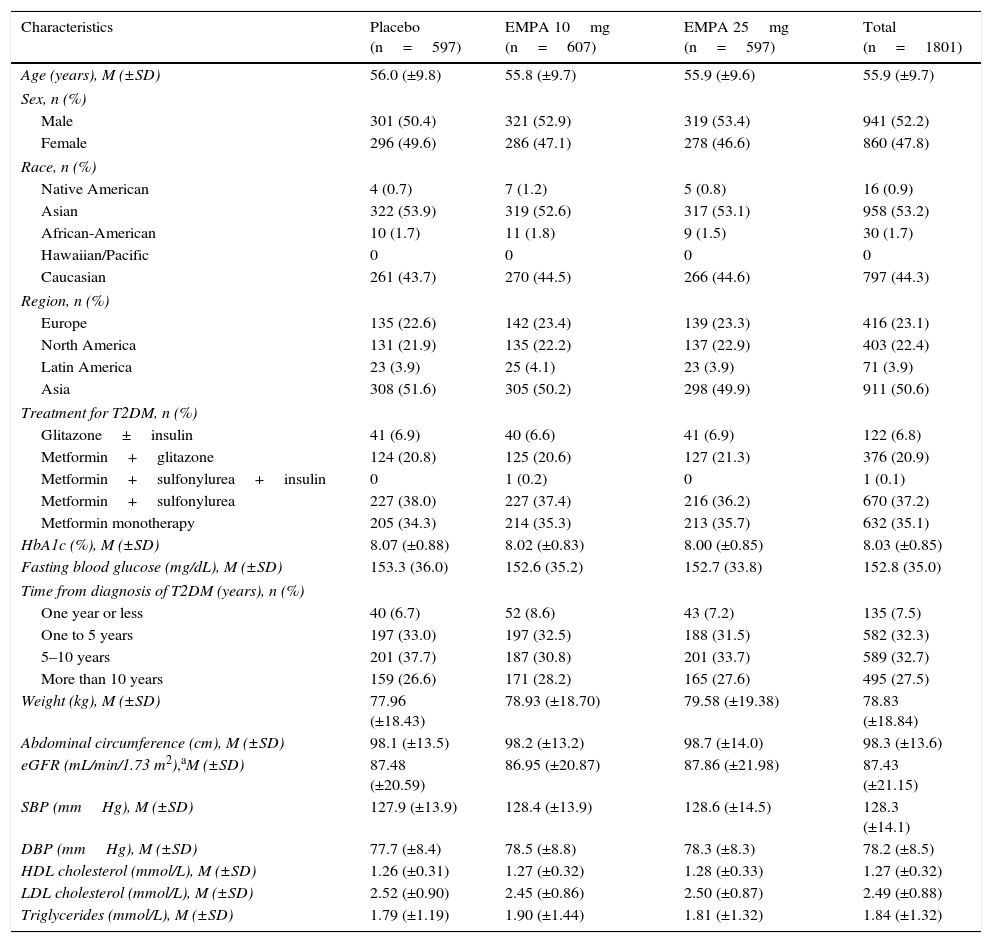
Demographic and clinical characteristics of recruited patients were similar in all treatment groups (Table 1). Males accounted for 52.2% of total population, mean patient age was 55.9±9.7 years (mean±SD; range: 19–85), and mean baseline HbA1c was 8.03±0.85%. Most patients received metformin as underlying treatment either as monotherapy (35.1%; n=632) or combined with a sulfonylurea (37.3%, n=671) or pioglitazone (20.9%; n=376). T2DM had been diagnosed at least five years before study entry in 60.2% of patients (n=1084).
Demographic and baseline characteristics of the population (CAS).
| Characteristics | Placebo (n=597) | EMPA 10mg (n=607) | EMPA 25mg (n=597) | Total (n=1801) |
|---|---|---|---|---|
| Age (years), M (±SD) | 56.0 (±9.8) | 55.8 (±9.7) | 55.9 (±9.6) | 55.9 (±9.7) |
| Sex, n (%) | ||||
| Male | 301 (50.4) | 321 (52.9) | 319 (53.4) | 941 (52.2) |
| Female | 296 (49.6) | 286 (47.1) | 278 (46.6) | 860 (47.8) |
| Race, n (%) | ||||
| Native American | 4 (0.7) | 7 (1.2) | 5 (0.8) | 16 (0.9) |
| Asian | 322 (53.9) | 319 (52.6) | 317 (53.1) | 958 (53.2) |
| African-American | 10 (1.7) | 11 (1.8) | 9 (1.5) | 30 (1.7) |
| Hawaiian/Pacific | 0 | 0 | 0 | 0 |
| Caucasian | 261 (43.7) | 270 (44.5) | 266 (44.6) | 797 (44.3) |
| Region, n (%) | ||||
| Europe | 135 (22.6) | 142 (23.4) | 139 (23.3) | 416 (23.1) |
| North America | 131 (21.9) | 135 (22.2) | 137 (22.9) | 403 (22.4) |
| Latin America | 23 (3.9) | 25 (4.1) | 23 (3.9) | 71 (3.9) |
| Asia | 308 (51.6) | 305 (50.2) | 298 (49.9) | 911 (50.6) |
| Treatment for T2DM, n (%) | ||||
| Glitazone±insulin | 41 (6.9) | 40 (6.6) | 41 (6.9) | 122 (6.8) |
| Metformin+glitazone | 124 (20.8) | 125 (20.6) | 127 (21.3) | 376 (20.9) |
| Metformin+sulfonylurea+insulin | 0 | 1 (0.2) | 0 | 1 (0.1) |
| Metformin+sulfonylurea | 227 (38.0) | 227 (37.4) | 216 (36.2) | 670 (37.2) |
| Metformin monotherapy | 205 (34.3) | 214 (35.3) | 213 (35.7) | 632 (35.1) |
| HbA1c (%), M (±SD) | 8.07 (±0.88) | 8.02 (±0.83) | 8.00 (±0.85) | 8.03 (±0.85) |
| Fasting blood glucose (mg/dL), M (±SD) | 153.3 (36.0) | 152.6 (35.2) | 152.7 (33.8) | 152.8 (35.0) |
| Time from diagnosis of T2DM (years), n (%) | ||||
| One year or less | 40 (6.7) | 52 (8.6) | 43 (7.2) | 135 (7.5) |
| One to 5 years | 197 (33.0) | 197 (32.5) | 188 (31.5) | 582 (32.3) |
| 5–10 years | 201 (37.7) | 187 (30.8) | 201 (33.7) | 589 (32.7) |
| More than 10 years | 159 (26.6) | 171 (28.2) | 165 (27.6) | 495 (27.5) |
| Weight (kg), M (±SD) | 77.96 (±18.43) | 78.93 (±18.70) | 79.58 (±19.38) | 78.83 (±18.84) |
| Abdominal circumference (cm), M (±SD) | 98.1 (±13.5) | 98.2 (±13.2) | 98.7 (±14.0) | 98.3 (±13.6) |
| eGFR (mL/min/1.73 m2),aM (±SD) | 87.48 (±20.59) | 86.95 (±20.87) | 87.86 (±21.98) | 87.43 (±21.15) |
| SBP (mmHg), M (±SD) | 127.9 (±13.9) | 128.4 (±13.9) | 128.6 (±14.5) | 128.3 (±14.1) |
| DBP (mmHg), M (±SD) | 77.7 (±8.4) | 78.5 (±8.8) | 78.3 (±8.3) | 78.2 (±8.5) |
| HDL cholesterol (mmol/L), M (±SD) | 1.26 (±0.31) | 1.27 (±0.32) | 1.28 (±0.33) | 1.27 (±0.32) |
| LDL cholesterol (mmol/L), M (±SD) | 2.52 (±0.90) | 2.45 (±0.86) | 2.50 (±0.87) | 2.49 (±0.88) |
| Triglycerides (mmol/L), M (±SD) | 1.79 (±1.19) | 1.90 (±1.44) | 1.81 (±1.32) | 1.84 (±1.32) |
T2DM, type 2 diabetes mellitus; EMPA, empagliflozin; SD, standard deviation; CAS, complete analysis set; HDL, high density lipoprotein; LDL, low density lipoprotein; M, mean; DBP, diastolic blood pressure; SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate.
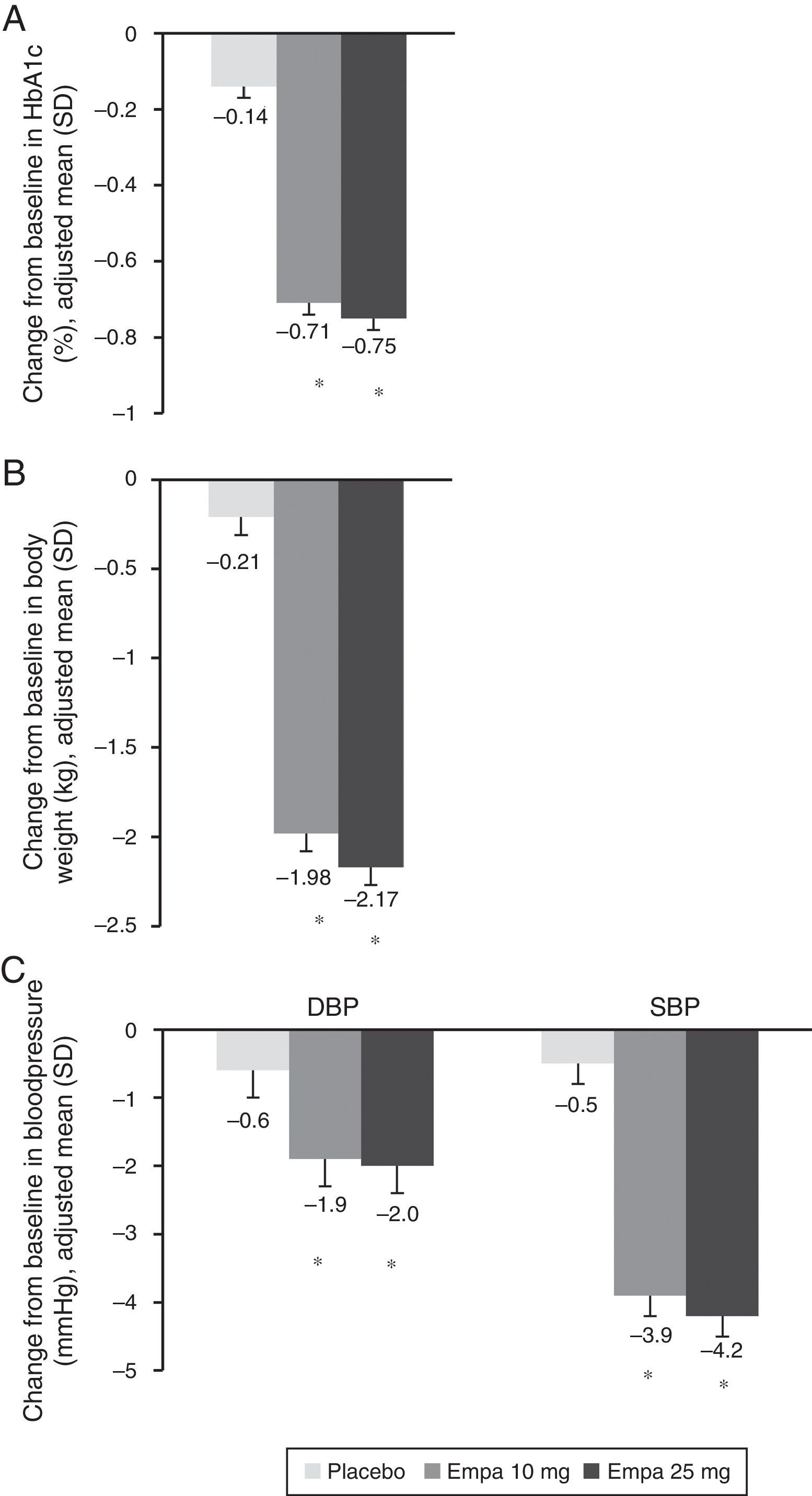
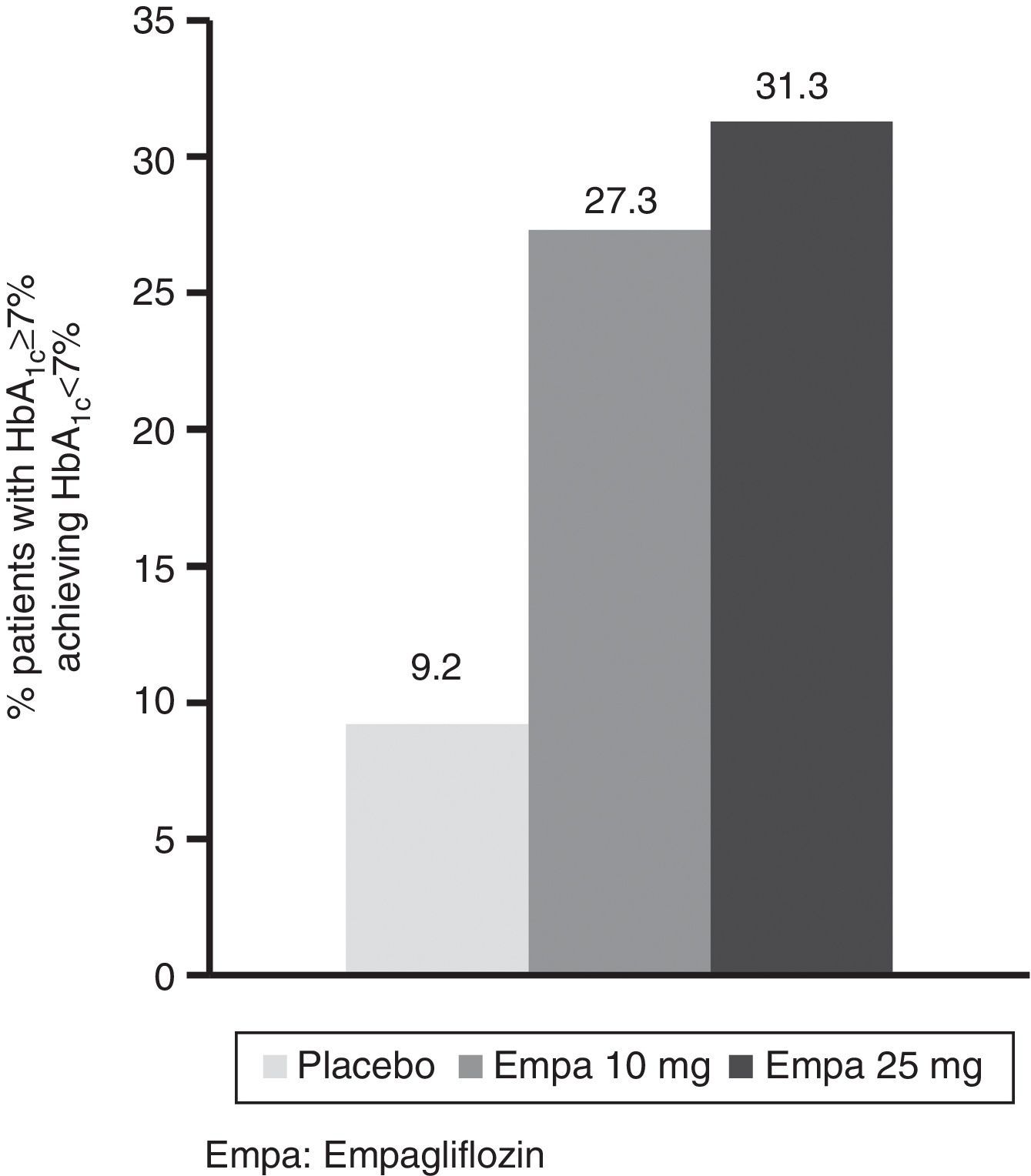
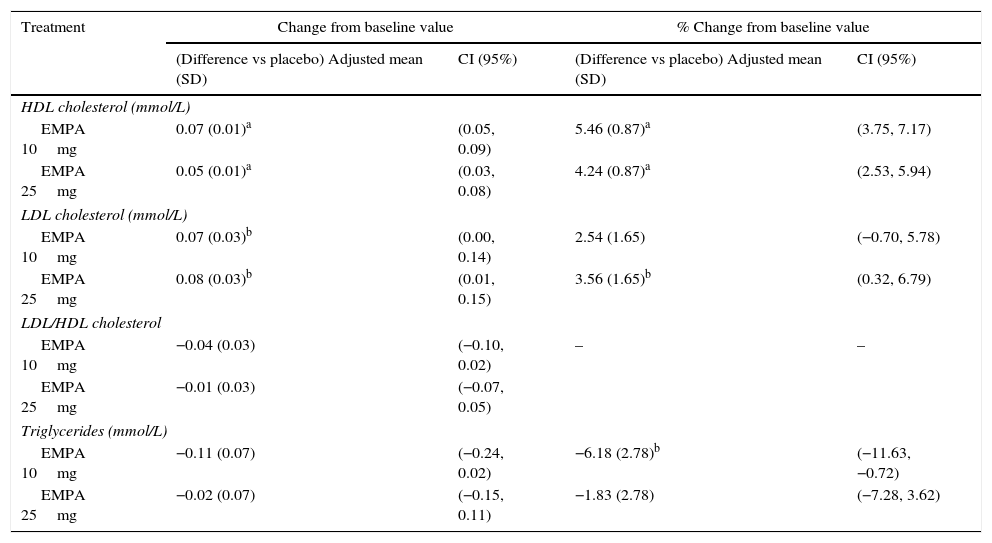
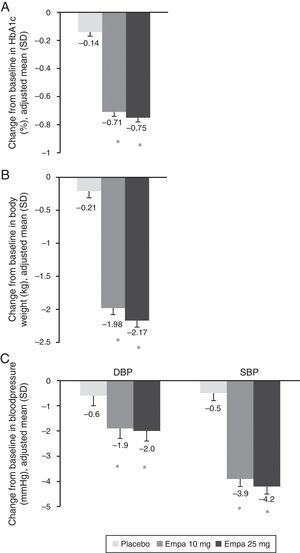
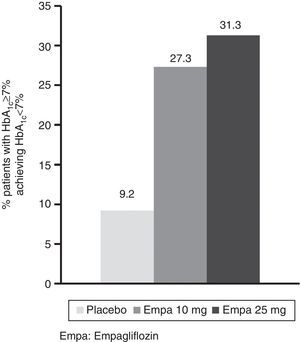
HbA1c levels significantly decreased at 24 weeks of treatment as compared to baseline in both treatment groups with empagliflozin (10 and 25mg) vs placebo (Fig. 1A). The difference in mean adjusted value was −0.58% ([95% CI: −0.66; −0.49], p<0.0001) between placebo and empagliflozin 10mg and −0.62% ([95% CI: −0.70; −0.53], p<0.0001) between placebo and empagliflozin 25mg. In patients with baseline HbA1c levels 7% or higher, the proportion achieving values lower than 7% was greater with empagliflozin 10mg and 25mg (27.3% and 31.3% respectively) as compared to placebo (9.2%) (Fig. 2).
Change in HbA1c (A), weight (B), and SBP and DBP (C) in the overall population 24 weeks after study start. Results are given as change in adjusted mean (ANCOVA), together with standard deviation and calculated p-value. Empa, emplagliflozin; DBP, diastolic blood pressure; SBP, systolic blood pressure. *p<0.001 (difference vs placebo).
When both empagliflozin doses were compared to placebo, a statistically significant decrease was seen in body weight (Fig. 1B), SBP, and DBP (Fig. 1C). The difference in mean adjusted weight as compared to placebo was −1.77kg ([95% CI: −2.05; −1.48], p<0.0001) with empagliflozin 10mg and −1.96kg ([95% CI: −2.24; −1.67], p<0.0001) with empagliflozin 25mg. Reduction in mean adjusted SBP vs placebo was 3.5mmHg with empagliflozin 10mg ([95% CI: −4.7; −2.3], p<0.0001) and 3.8mmHg ([95% CI: −5.0; −2.6], p<0.0001) with empagliflozin 25mg. Reduction in mean DBP vs placebo was in turn 1.3mmHg with empagliflozin 10mg ([95% CI: −2.1; −0.5], p<0.001) and 1.4mmHg with empagliflozin 25mg ([95% CI: −2.1; −0.6], p<0.001).
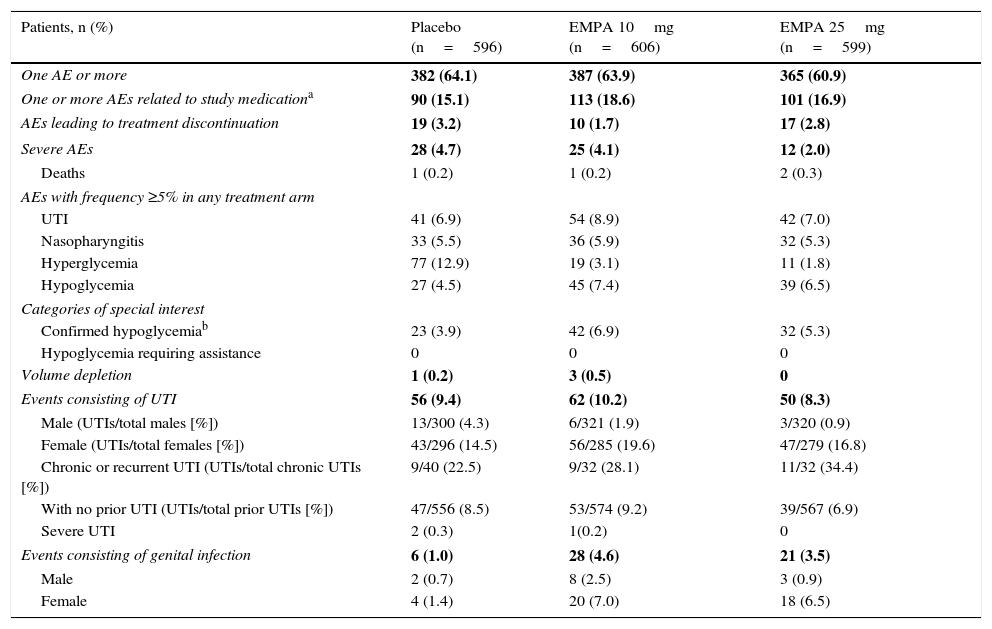
SafetyIncidence of one or more AEs was similar in all three treatment arms of this study (Table 2). The proportion of patients who discontinued treatment for AEs was slightly lower with empagliflozin 10mg (1.7%) as compared to empagliflozin 25mg (2.8%) and placebo (3.2%).
Summary of adverse effects (TP).
| Patients, n (%) | Placebo (n=596) | EMPA 10mg (n=606) | EMPA 25mg (n=599) |
|---|---|---|---|
| One AE or more | 382 (64.1) | 387 (63.9) | 365 (60.9) |
| One or more AEs related to study medicationa | 90 (15.1) | 113 (18.6) | 101 (16.9) |
| AEs leading to treatment discontinuation | 19 (3.2) | 10 (1.7) | 17 (2.8) |
| Severe AEs | 28 (4.7) | 25 (4.1) | 12 (2.0) |
| Deaths | 1 (0.2) | 1 (0.2) | 2 (0.3) |
| AEs with frequency ≥5% in any treatment arm | |||
| UTI | 41 (6.9) | 54 (8.9) | 42 (7.0) |
| Nasopharyngitis | 33 (5.5) | 36 (5.9) | 32 (5.3) |
| Hyperglycemia | 77 (12.9) | 19 (3.1) | 11 (1.8) |
| Hypoglycemia | 27 (4.5) | 45 (7.4) | 39 (6.5) |
| Categories of special interest | |||
| Confirmed hypoglycemiab | 23 (3.9) | 42 (6.9) | 32 (5.3) |
| Hypoglycemia requiring assistance | 0 | 0 | 0 |
| Volume depletion | 1 (0.2) | 3 (0.5) | 0 |
| Events consisting of UTI | 56 (9.4) | 62 (10.2) | 50 (8.3) |
| Male (UTIs/total males [%]) | 13/300 (4.3) | 6/321 (1.9) | 3/320 (0.9) |
| Female (UTIs/total females [%]) | 43/296 (14.5) | 56/285 (19.6) | 47/279 (16.8) |
| Chronic or recurrent UTI (UTIs/total chronic UTIs [%]) | 9/40 (22.5) | 9/32 (28.1) | 11/32 (34.4) |
| With no prior UTI (UTIs/total prior UTIs [%]) | 47/556 (8.5) | 53/574 (9.2) | 39/567 (6.9) |
| Severe UTI | 2 (0.3) | 1(0.2) | 0 |
| Events consisting of genital infection | 6 (1.0) | 28 (4.6) | 21 (3.5) |
| Male | 2 (0.7) | 8 (2.5) | 3 (0.9) |
| Female | 4 (1.4) | 20 (7.0) | 18 (6.5) |
AE, adverse effect; EMPA, empagliflozin; UTI, urinary tract infection; TP, treated population.
Incidence of UTIs was similar in the placebo and empagliflozin 25mg groups (6.9% and 7.0% respectively), but higher in patients treated with empagliflozin 10mg (8.9%). In all three treatment arms, UTIs were more common in females as compared to males, and also in patients with chronic or recurrent UTI as compared to those with no prior UTI. The vast majority of UTIs recorded were mild in nature. Only two patients given placebo and one receiving empagliflozin 10mg reported severe UTIs.
More patients experienced genital infection with empagliflozin 10mg and 25mg as compared to placebo (4.6% and 3.5% vs 1%). Genital infections were reported in more women than men treated with empagliflozin (7.0% and 2.5% for empagliflozin 10mg respectively; 6.5% and 0.9% for empagliflozin 25mg respectively). All events were mild to moderate in severity, and the drug was not discontinued in most cases. Confirmed hypoglycemia rates were higher in patients treated with empagliflozin than in placebo patients (6.9%, 5.3%, and 3.9% with empagliflozin 10mg and 25mg and placebo respectively), and hypoglycemia predominantly occurred in patients treated with sulfonylureas. No severe episode or event requiring assistance was recorded.
Table 3 summarizes the effects of lipid profile. For HDL cholesterol, the mean adjusted percent change from baseline was +5.57% with empagliflozin 10mg, +4.35% with empagliflozin 25mg, and +0.11% with placebo (p<0.0001). The corresponding values for LDL cholesterol were +6.75% and +7.76% with empagliflozin 10mg and 25mg respectively and +4.21% with placebo (p=0.031 for empagliflozin 25mg vs placebo). There were no significant changes vs placebo in mean adjusted values of the LDL/HDL cholesterol ratio or triglyceride levels.
Changes in lipid parameters with empagliflozin compared to placebo (TP).
| Treatment | Change from baseline value | % Change from baseline value | ||
|---|---|---|---|---|
| (Difference vs placebo) Adjusted mean (SD) | CI (95%) | (Difference vs placebo) Adjusted mean (SD) | CI (95%) | |
| HDL cholesterol (mmol/L) | ||||
| EMPA 10mg | 0.07 (0.01)a | (0.05, 0.09) | 5.46 (0.87)a | (3.75, 7.17) |
| EMPA 25mg | 0.05 (0.01)a | (0.03, 0.08) | 4.24 (0.87)a | (2.53, 5.94) |
| LDL cholesterol (mmol/L) | ||||
| EMPA 10mg | 0.07 (0.03)b | (0.00, 0.14) | 2.54 (1.65) | (−0.70, 5.78) |
| EMPA 25mg | 0.08 (0.03)b | (0.01, 0.15) | 3.56 (1.65)b | (0.32, 6.79) |
| LDL/HDL cholesterol | ||||
| EMPA 10mg | −0.04 (0.03) | (−0.10, 0.02) | – | – |
| EMPA 25mg | −0.01 (0.03) | (−0.07, 0.05) | ||
| Triglycerides (mmol/L) | ||||
| EMPA 10mg | −0.11 (0.07) | (−0.24, 0.02) | −6.18 (2.78)b | (−11.63, −0.72) |
| EMPA 25mg | −0.02 (0.07) | (−0.15, 0.11) | −1.83 (2.78) | (−7.28, 3.62) |
SD, standard deviation; EMPA, empagliflozin; HDL, high density lipoprotein; CI, confidence interval; LDL, low density lipoprotein.
This post hoc analysis based on three Phase III studies shows that empagliflozin, in combination with other oral glucose-lowering drugs, effectively decreases HbA1c levels in patients with T2DM previously treated with monotherapy or dual therapy who have an inadequate blood glucose control. Treatment with empagliflozin was also associated to decreased body weight and blood pressure, and showed a good safety and tolerability profile. These data support empagliflozin as an adequate therapeutic option for second or third-line treatment of T2DM.
In this analysis, treatment with empagliflozin decreased HbA1c by 0.57–0.61% and weight by 1.77–1.96kg. SBP and DBP were also decreased as compared to placebo. These findings are consistent with those previously reported with SGLT2 inhibitors.14–17
The hypoglycemic mechanism of action of empagliflozin is reduction of glucose reabsorption. The amount of glucose excreted through the kidney by this mechanism depends on blood glucose levels. Patients with higher plasma glucose levels therefore have a greater urinary excretion of glucose.18 This is why the most marked HbA1c decreases occur in patients with poorer control.11 On the other hand, empagliflozin efficacy did not decrease in the 60% of patients with a time since T2DM onset ≥5 years and, thus, greater pancreatic β-cell dysfunction.6 The mechanism of action independent from insulin secretion would account for persistence of the hypoglycemic efficacy of empagliflozin in the more advanced stages of T2DM, an aspect that differentiates this drug from other hypoglycemic drug classes.
The safety and tolerability profile of empagliflozin was good, thus supporting the data from other studies. AE rate was similar in the placebo and empagliflozin (10mg and 25mg) treatment arms. As expected, genital infections were more frequent in patients treated with empagliflozin, being more prevalent in females and in patients with history of recurrent candidiasis.19,20 Most ITUs recorded were mild, and their incidence was only slightly higher with empagliflozin 10mg, but similar in patients treated with empagliflozin 25mg and placebo. Although SGLT2 inhibitors do not intrinsically cause hypoglycemia,9,16,17 incidence of hypoglycemic episodes may increase when blood glucose control is optimized, particularly when they are combined with sulfonylureas or other drugs inducing hypoglycemia.21–23 However, this analysis did not find hypoglycemic episodes that were severe or required assistance. Finally, increases in HDL cholesterol and, to a lesser extent, LDL cholesterol seen with empagliflozin are consistent with those reported in prior studies on SGLT2 inhibitors.24,25 The pathophysiology of these changes is unknown, and their clinical significance has not been elucidated yet, but they do not modify the atherogenic LDL/HDL cholesterol index.
One of the limitations of this analysis is that it was a post hoc analysis. In addition, results are only applicable to patients with the characteristics of the population participating in the clinical trials on which the analysis is based. In this regard, it would be interesting to test the effects derived from addition of empagliflozin to the treatment of patients with T2DM with less restrictions. It should also be noted that studies only lasted 24 weeks, so that durability of the effects reported may not be amenable to extrapolation. On the other hand, the large sample size of this analysis confers more strength to its conclusions.
Today, in most patients with T2DM, failure of metformin monotherapy2–4 requires progression to treatment with two or even three drugs,1 which may result, depending on the agent selected, in an additional risk of hypoglycemia or weight increase.5 In this regard, the results reported in this study support the efficacy of treatment with empagliflozin combined with other oral antidiabetics for decreasing HbA1c, body weight, and blood pressure with a good overall safety and tolerability profile.
Ethical disclosuresProtection of human and animal subjectsThe authors state that no experiments with humans or animals have been conducted in this research. The authors state that all procedures used in the reported studies met the regulations of the relevant ethics research committee and the World Medical Assembly and the Declaration of Helsinki.
Confidentiality of dataNot applicable.
Right to privacy and informed consentNot applicable.
FundingParticipation of Boehringer Ingelheim in studies included in this analysis consisted of study design and data collection and analysis. Involvement of Eli Lilly and Company was limited to co-funding of these studies. Boehringer Ingelheim and Eli Lilly and Company participated in analyses and preparation of this manuscript.
AuthorshipDr. Irene Romera, Dr. Bernat Ariño, Dr. Francisco Javier Ampudia-Blasco, and Dr. Antonio Pérez participated in conception and design of the manuscript and data interpretation. Egon Pfarr, Dr. Sanja Giljanovic Kis, and Dr. Ebrahim Naderali participated in analysis and interpretation of results. All authors participated in preparation, revision, and approval of the manuscript submitted.
Conflicts of interestIrene Romera, Sanja Giljanovic Kis, and Ebrahim Naderali are full-time employees at Eli Lilly and Company. Bernat Ariño and Egon Pfarr are full-time employees at Boehringer Ingelheim. Francisco Javier Ampudia-Blasco and Antonio Pérez have received fees from Boehringer-Ingelheim and Eli Lilly and Company for consultancy and lectures.
We thank BCNscience, S.L. for assistance in medical writing.
Please cite this article as: Romera I, Ampudia-Blasco FJ, Pérez A, Ariño B, Pfarr E, Giljanovic Kis S, et al. Eficacia y seguridad de empagliflozina en combinación con otros hipoglucemiantes orales en pacientes con diabetes mellitus tipo 2. Endocrinol Nutr. 2016;63:519–526.