Lipid emulsions incorporated into parenteral nutrition (PN) make it possible to provide essential fatty acids and to cover energy requirements without excessive carbohydrate content. On the other hand, both the composition of these emulsions and the dose supplied are related to the development of liver disease.1,2
Several articles on the effect of intravenous lipid emulsions based on omega-3 fatty acids (W3FA) alone in the treatment of liver disease in adults have recently been published.3–7
We report the case of an adult patient with the longest treatment period reported in Spain who showed evidence of laboratory and functional improvement with this type of lipid emulsion.
This was a 44-year-old female patient on PN since 2010 after massive bowel resection for a desmoid tumor which left a residual bowel consisting of 12cm of duodenum, half the transverse colon, and the left colon. In addition to cyclic nutritional support, she was given from the start treatment with ursodeoxycholic acid and antibiotics for bacterial overgrowth to prevent the development of liver disease. The length of the remaining small bowel precluded enteral nutrition.
During 2010 and 2011, the lipid formula administered consisted of medium-chain triglycerides (MCTs), soybean oil, and W3FA (5:4:1 ratio) at doses of 1.1–1.4g/kg/day, carbohydrate:lipids ratio 60:40, and a nitrogen solution with a high concentration of branched amino acids (16g nitrogen/day). In view of liver enzyme elevation, trace elements were replaced in October 2011 by another product with a lower manganese content, which resulted in liver function improvement at the end of 2011.
After work-up at a reference hospital for bowel transplant, a liver biopsy was performed in April 2012. It revealed parenchymal and canalicular cholestasis in the liver parenchyma consistent with the changes induced by PN. The bilirubin level increased from 5.1mg/dL to 15.4mg/dL after the liver biopsy (complicated by a hematoma and hemoperitoneum). Because of this sudden liver function impairment, lipid administration was suspended for 3 weeks, but was restarted in June using a different lipid emulsion (a physical mixture of soybean oil, MCTs, olive oil, and W3FA in a 3:3:2.5:1.5 ratio) at a dose of 25g/day, which was gradually increased in the following months up to 50g/48h. Partial improvement of the bilirubin level was found by the end of the year. When lipid provision decreased, the patient required supplementation with vitamins K and A.
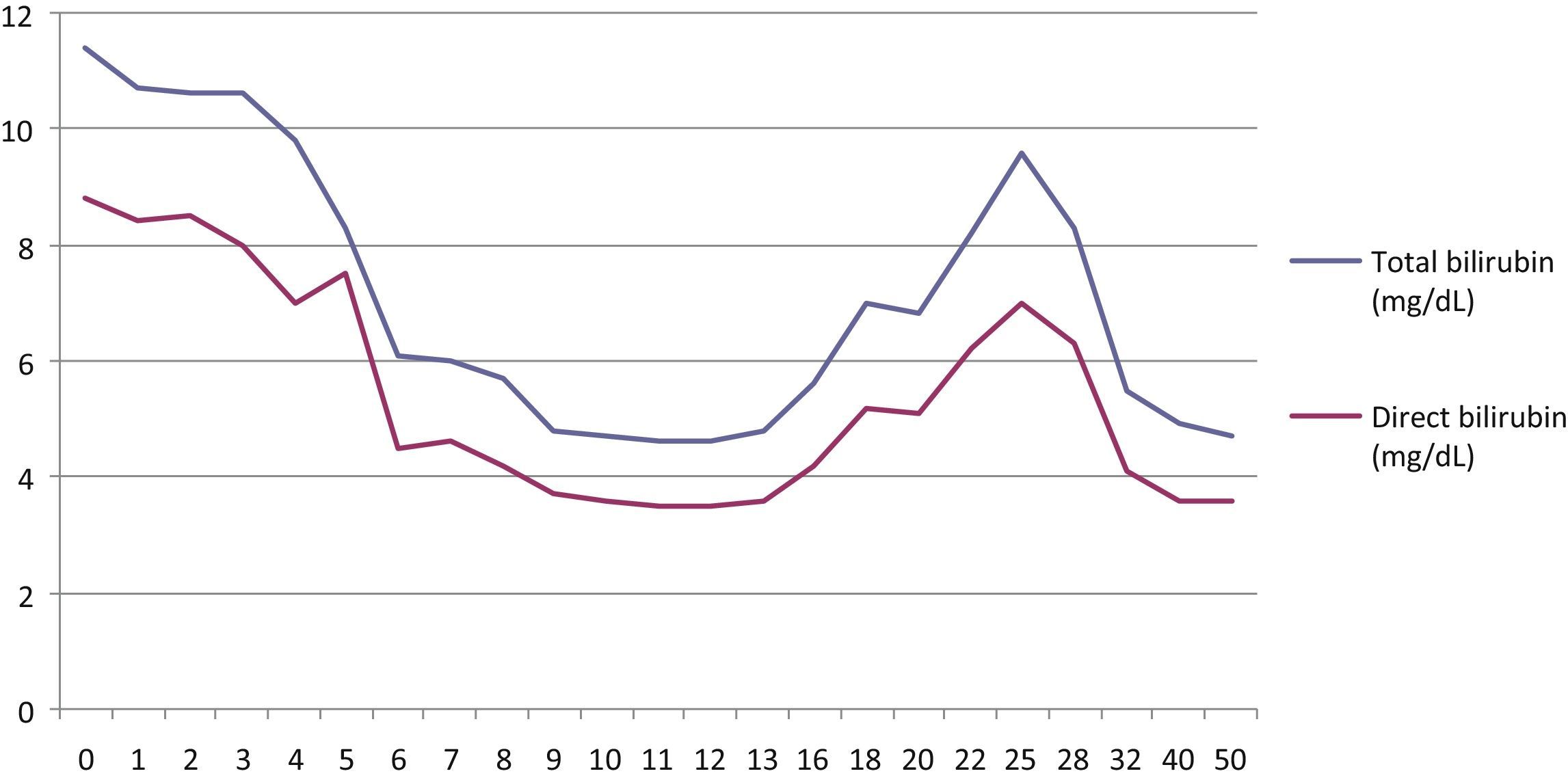
During 2013, because of liver function improvement and progressive weight loss (lowest weight achieved in April, 44.5kg; BMI 16.6kg/m2), lipid provision was increased up to a maximum of 65g/day for 4 days per week and 40g/day on the other days (Fig. 1).
Regular ultrasound examinations performed during follow-up showed a liver of normal size, morphology and echogenicity, splenomegaly, and biliary sludge. In October 2013, ultrasound examination showed evidence of portal hypertension (with no evidence of esophageal varices in gastroscopy) with a slightly enlarged portal vein with hepatofugal flow. Fibroscan® tests performed in 2012 and 2013 revealed no evidence of liver fibrosis (stiffness 4.8kPa, equivalent to fibrosis levels of 0–1 in biopsy).
In March 2014, the patient again experienced progressive liver function impairment (maximum total bilirubin 11.4mg/dL). After other causes of liver disease had been ruled out, the patient rejected the option of a bowel transplant, and the literature was reviewed.3–6 She was then offered a replacement of the lipid formula by another containing W3FA only (45g/day, 0.9g/kg, carbohydrates:lipids 66:33; 1605 calories). This type of lipid emulsion is approved, but not marketed, in Spain for concomitant use with another lipid emulsion at a maximum dose of 1–2mL/kg. Authorization was requested from the Spanish Agency for Medicinal Products and the hospital management for off-label use, as in the reports reviewed (lipid emulsion alone), and the patient gave her written consent for the treatment.
In the first 4 weeks of treatment, the patient experienced side effects which have been reported as uncommon in the prescribing information of the product (headache, asthenia, hyperthermia, nausea, abdominal fullness), which resolved spontaneously. At 8 weeks, the total bilirubin level had decreased by 50% (5.7mg/dL), and it reached a minimum value at week 12 (4.6mg/dL). Bilirubin increased again in week 18, and it was therefore decided to administer the same dose only every other day (45g/day, 3 days weekly). This dosage is still being administered to the patient, who weighs 48.4kg and has a BMI of 18.1kg/m2. At the time of the submission of this article, the total bilirubin level was less than 5mg/dL at week 50 of treatment with Omegaven®, with a progressive improvement in cholestasis.
A repeat ultrasound examination 2 months after treatment start showed a portal vein in the upper limit of normal with preserved hepatopetal flow (found again in the ultrasound examination performed in week 28 of treatment).
A Fibroscan® performed in September 2014 showed no evidence of liver fibrosis (stiffness 4.6kPa).
Since the administration of a lipid formula containing only W3FA was started, essential fatty acids have regularly been measured. Linoleic acid was below the normal limit in two measurements, but its metabolites (γ-linoleic and arachidonic acids) were within the normal range.
In conclusion, treatment with an intravenous lipid emulsion containing only W3FA was found to significantly improve total and direct bilirubin levels, with an even more striking functional improvement (a change in the direction of portal flow in ultrasound examination as indirect evidence of portal hypertension improvement). Ideally, these results ought to be confirmed by a repeat liver biopsy. However, because of her history and the little impact its result would have on the current treatment scheme, the patient does not contemplate this option.
Further studies on larger patient samples and for long periods are needed to verify whether the greater or lesser proportion of omega-3 and omega-6 acids in the lipid emulsion used in PN has a determinant influence on the course/reversal of liver disease.
Conflict of interestThe authors state that they have no conflicts of interest.
To nurse Susana Arnedo and Dr. Elena Otero, from Estella Healthcare Center, and to Dr. Santiago Ostiz, from the radiology department of Complejo Hospitalario de Navarra, for their availability and collaboration in the regular follow-up of the patient.
Please cite this article as: Zugasti Murillo A, Petrina Jáuregui E, Elizondo Armendáriz J. Emulsión lipídica exclusiva de ácidos grasos omega-3 (Omegaven®) en el tratamiento de la hepatopatía asociada a nutrición parenteral: a propósito de un caso. Endocrinol Nutr. 2015;62:294–296.