The histological and immunohistochemical profile of medullary thyroid carcinoma is ill-defined. The objective of this study was to determine the epidemiological, histological, and immunohistochemical characteristics of medullary carcinoma and to analyze whether differences exist between sporadic and familial carcinomas.
Patients and methodsFifty-five histologically confirmed tumors were studied. Histological slides were reviewed and immunohistochemical staining of the archival paraffin blocks was performed.
ResultsNineteen of the 55 carcinomas (35%) were sporadic, and 36 (65%) familial. Sex distribution was similar, but familial carcinoma was more common in patients under 40 years of age (p<0.001). A solid growth pattern and plasmacytoid cells were found in most cases. C-cell hyperplasia and multicentricity were more frequent findings in familial carcinoma, while tumor necrosis, hemorrhagic foci, vascular invasion, and neovascularization were more common in the sporadic type. Immunohistochemical staining was positive for calcitonin, CEA, bcl-2, and p53 protein. With regard to staging, familial carcinomas were diagnosed in the earliest stages, when they were smaller and there were no lymph node metastases (p<0.01).
ConclusionsFamilial cases were more frequent when there was more C-cell hyperplasia and multicentricity. Sporadic cases more frequently showed foci of necrosis, hemorrhage, vascular invasion, and neovascularization. Neither histopathological nor immunohistochemical criteria are useful for differentiating between familial and sporadic forms.
En el carcinoma medular tiroideo (CMT) el perfil histológico e inmuno-histoquímico está mal definido. El objetivo de este estudio es determinar las características clínicas, histológicas e inmuno-histoquímicas del CMT, y analizar si existen diferencias entre los carcinomas esporádicos y familiares.
Material y métodoSe han incluido 55 tumores confirmados histológicamente. Fueron revisadas las preparaciones histológicas y se llevaron a cabo las tinciones de inmuno-histoquímica de los bloques de parafina del archivo.
ResultadosDe los 55 carcinomas, 19 (35%) fueron esporádicos y 36 (65%) de tipo familiar. La distribución por sexo es similar, sin embargo, los carcinomas familiares tienen una mayor frecuencia de pacientes menores de 40 años (p<0,001). En la mayor parte de los casos se ha observado un patrón de crecimiento sólido y el tipo celular plasmocitoide. Son hallazgos más frecuentes en el familiar la hiperplasia de células C y la multicentricidad, mientras que en el esporádico es más frecuente la necrosis tumoral, los focos hemorrágicos, la invasión vascular y la presencia de neovascularización. Respecto a la inmuno-histoquímica, los tumores muestran positividad intensa con marcadores para calcitonina, CEA y bcl-2, y proteína p53. En cuanto al estadiaje, los carcinomas familiares son diagnósticados en estadios más iniciales, con tamaño más pequeño y sin metástasis ganglionares (p<0,01).
ConclusionesLos CMT familiares presentan con más frecuencia hiperplasia de células C y multicentricidad, y los esporádicos muestran con más frecuencia focos de necrosis, hemorragia, invasión vascular y neovascularización. Ni los criterios histológicos ni los inmuno-histoquímicos son arquitecturales para diferenciar las formas familiar y esporádica.
Thyroid tumor pathology is diverse1,2 and often poses a significant diagnostic challenge.3,4 The most common thyroid carcinoma is differentiated carcinoma, which has a rather favorable prognosis when managed with surgery and iodine therapy.5 Medullary thyroid carcinoma (MTC) is an uncommon condition (3–10% of thyroid neoplasms) arising from parafollicular C cells and may be sporadic or familial in nature. Familial MTC, which is associated with a characteristic phenotype, is the predominant form.6–8
MTC is an uncommon tumor and thus difficult to study because of the limited number of patients and series.9 In addition, from the histological viewpoint, MTC may show a great variability in its cytoarchitectural characteristics, which makes it difficult to recognize and may require the use of immunochemistry techniques for diagnosis.9,10 There is also no agreement regarding the classification of these tumors into different cytoarchitectural patterns.9 Thus, studies such as the one by Franc et al.11 take into account up to 8 architectural and 9 cellular features, while other studies consider a lower number of characteristics.12–14
However, the immunohistochemical profile of these tumors is poorly known because of their uncommon occurrence and the heterogeneity of the methods used. Thus, assessment of results obtained with immunohistochemical staining is controversial, because not all authors agree as to how positivity should be quantified and there may be changes in the sensitivity of the different antisera used.9,13,15
The objective of this study was to analyze the clinical, histological, and immunohistochemical characteristics of MTC, and to find out whether clinical, morphological, and immunohistochemical differences exist between sporadic and familial carcinomas.
Patients and methodsSelection criteria of the study populationFifty-five patients undergoing surgery for MTC at the department of general and gastrointestinal surgery of Hospital Universitario Virgen de la Arrixaca from 1971 to 2004 and who met the following criteria were enrolled into the study:
- (1)
Histological documentation of medullary thyroid carcinoma.
- (2)
The availability of histological slides and paraffin blocks of the MTC resected during initial surgery for the tumor.
- (3)
The availability of a complete clinical history of the patient recording the minimal clinical and epidemiological data required by the study protocol.
Data on the clinical characteristics and course were retrospectively collected from the clinical histories.
Gross, microscopic, and immunohistochemical characteristics were collected by reviewing histological tumor slides, together with data available from the relevant pathological report. In addition, in order to complete the study, immunohistochemical staining was performed on the paraffin-embedded tumor blocks from each patient.
Description of the seriesAmong the 55 patients enrolled into the study, 54% were females (n=30) and 66% (n=36) were aged 40 years or less (mean age, 36 years). As to the type of carcinoma, 35% (n=19) were sporadic and the remaining 65% (n=36) occurred in families with the MEN 2A syndrome (of these, 29 and 7 had the Cys634Thyr and Cys634Arg mutations, respectively).
Basal calcitonin levels before surgery were 2690.2 (931)pg/ml, while calcitonin levels after stimulation were 8520.6 (1321)pg/ml, and CEA levels 16.3 (12)mcg/l.
All patients underwent total thyroidectomy. In addition, 29% (n=16) of patients underwent central lymph node dissection, 15% (n=7) central plus bilateral jugular lymph node dissection, and 5% (n=3) ipsilateral jugular lymph node dissection.
Study variablesStudy variables were grouped as:
- (1)
Epidemiological variables: (a) sex; (b) age: ≤40 and >40 years, and (c) type of carcinoma; sporadic or familial-MEN 2A syndrome.16,17
- (2)
Microscopic characteristics: (a) architectural pattern, based on the predominant arrangement of tumor cells: solid (cells arranged as a “sheet” forming no identifiable structures), trabecular (cells arranged as cord-like structures of variable thickness), and acinar (cells in small rounded groups); (b) cytological pattern, depending on the predominant tumor morphology: plasmacytoid (cells with abundant cytoplasm and eccentric nuclei), fusocellular (elongated spindle-shaped cells), small cell (cells with hyperchromatic nuclei and scant cytoplasm), oncocytic (cells with very abundant, strongly eosinophilic cytoplasm), and clear cell tumors (cells with very abundant, optically clear cytoplasm); (c) amyloid, as shown by Congo red staining; (d) collagen; (e) calcification, either of a dystrophic type or as psammoma bodies; (e) C-cell hyperplasia, defined as the presence of at least 50 C cells per low power field18; (f) multicentricity, depending on the presence or absence of more than one grossly recognized tumor nodule, and (g) other microscopic characteristics of malignant tumors: necrosis (gross or microscopic areas of tumor necrosis), mitosis (mitotic figures in at least 15% of tumor cells), bleeding (gross or microscopic hemorrhagic areas), vascular invasion (tumor cells in vessel lumen or wall), and neovascularization (the striking presence of newly formed vessels in the tumor).
- (3)
Immunohistochemical characteristics. Initial slides were reviewed, and new sections were made from paraffin blocks. Once tumors were stained with each of the markers, the semiquantitative Bergholm et al.15 method was used to classify them as low immunoreactivity (less than 10% of cells stained), medium immunoreactivity (10–15% of cells stained), and high immunoreactivity (more than 50% of cells stained) tumors based on their marker affinity. The immunohistochemical characteristics tested included: (a) calcitonin (measured using the DAKO Rabbit Anti-Human Calcitonin primary antibody); (b) CEA (DAKO Rabbit Anti-Human CEA primary antibody); (c) Bcl-2 (the primary antibody used was DAKO ChemMate Mouse Anti-Human BCL-2 Oncoprotein); (d) p-53 (DAKO ChemMate Mouse Anti-Human p53 Protein primary antibody); (e) Ki-67 (DAKO Rabbit Anti-Human Ki-67 Antigen primary antibody), and (f) C-erb-B2 (DAKO Rabbit Anti-Human C-erb-B2 Oncoprotein primary antibody).
- (4)
Staging and clinical course characteristics. (a) Stage, based on the 2002 TNM classification, as updated in 2009,19 was classified as stage I (tumor≤2cm limited to thyroid gland, with no lymph node or distant metastases [T1,N0,M0]); stage II (tumor>2cm but <4cm limited to thyroid gland, with no lymph node or distant metastases [T2,N0,M0]); stage III (tumor>4cm limited to thyroid gland or tumor with minimal extrathyroid extension and no lymph node or distant metastases [T3,N0,M0]; or any of the previously described three types [T1, T2, or T3] showing level VI lymph node metastases [pretracheal, paratracheal, prelaryngeal, Delphian lymph nodes, N1a]); and stage IV (T1, T2, or T3 with upper lymph node metastases, uni- or bilateral cervical bone involvement, N1b, or distant metastasis, M1; or T4a [a tumor of any size that grows outside the thyroid capsule and invades subcutaneous cell tissue, larynx, trachea, esophagus, of recurrent nerve]-T4b [a tumor invading the prevertebral fascia, carotid artery, or mediastinal vessels] with any N or M); (b) size (T1 [≤2cm], T2 [2–4cm], and T3 [>4cm]); (c) lymph node metastases, confirmed by histology; (d) distant metastasis; (e) lymph node recurrence, diagnosed by needle aspiration biopsy or increased serum calcitonin levels, and (f) local recurrence.
Data were processed using SPSS for Windows version 11.0 software. Descriptive statistics, a Chi-square test performed with residue analysis, and a Fisher test when required were done. A value of p<0.05 was considered statistically significant.
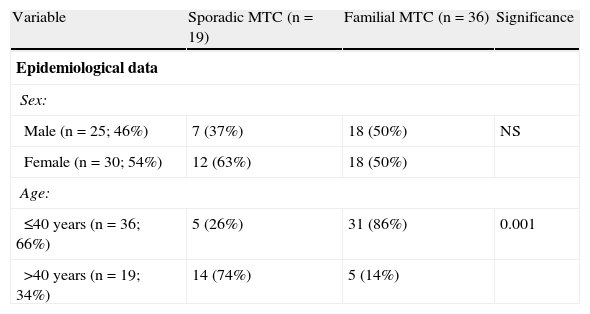
ResultsEpidemiological dataMean patient age was 36 years (6–83 years), and 54% of patients were female. Of the 55 carcinomas, 19 (35%) were sporadic and 36 (65%) familial. There was a slight female predominance (male:female ratio, 1:1.19) and a greater frequency of cases diagnosed and treated at a younger age (66% of patients were 40 years old or less) (Table 1).
Differences between sporadic and familial MTC.
| Variable | Sporadic MTC (n=19) | Familial MTC (n=36) | Significance |
| Epidemiological data | |||
| Sex: | |||
| Male (n=25; 46%) | 7 (37%) | 18 (50%) | NS |
| Female (n=30; 54%) | 12 (63%) | 18 (50%) | |
| Age: | |||
| ≤40 years (n=36; 66%) | 5 (26%) | 31 (86%) | 0.001 |
| >40 years (n=19; 34%) | 14 (74%) | 5 (14%) | |
MTC: medullary thyroid carcinoma; NS: not significant.
No significant difference was found in sex distribution between sporadic and familial tumors. As regards age, however, familial carcinomas were more common than sporadic tumors in patients under 40 years of age (86 versus 26%; p<0.001).
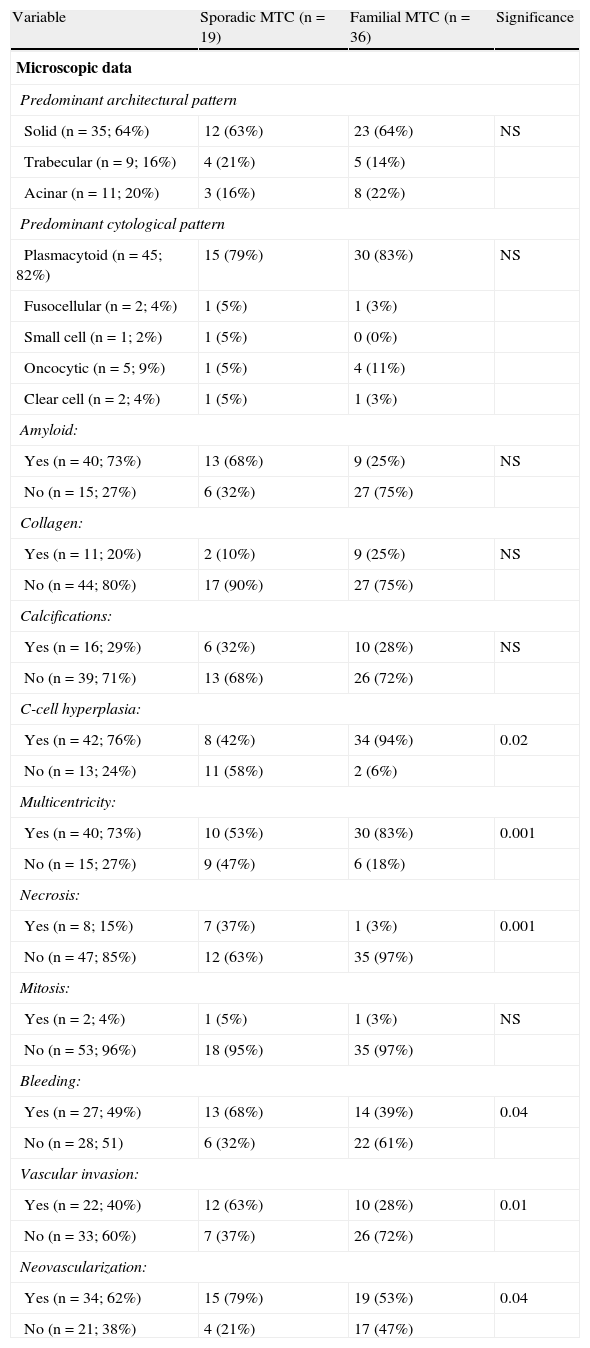
Microscopic characteristicsSolid growth was the predominant tumor pattern (64%), and a plasmacytoid cell type was the most common (82%). Amyloid substance was found in 73% of cases, while areas occupied by collagen material and calcification foci were identified in 20% of patients. No significant differences were shown for any of these variables between sporadic and familial MTC (Table 2).
Differences between sporadic and familial MTC.
| Variable | Sporadic MTC (n=19) | Familial MTC (n=36) | Significance |
| Microscopic data | |||
| Predominant architectural pattern | |||
| Solid (n=35; 64%) | 12 (63%) | 23 (64%) | NS |
| Trabecular (n=9; 16%) | 4 (21%) | 5 (14%) | |
| Acinar (n=11; 20%) | 3 (16%) | 8 (22%) | |
| Predominant cytological pattern | |||
| Plasmacytoid (n=45; 82%) | 15 (79%) | 30 (83%) | NS |
| Fusocellular (n=2; 4%) | 1 (5%) | 1 (3%) | |
| Small cell (n=1; 2%) | 1 (5%) | 0 (0%) | |
| Oncocytic (n=5; 9%) | 1 (5%) | 4 (11%) | |
| Clear cell (n=2; 4%) | 1 (5%) | 1 (3%) | |
| Amyloid: | |||
| Yes (n=40; 73%) | 13 (68%) | 9 (25%) | NS |
| No (n=15; 27%) | 6 (32%) | 27 (75%) | |
| Collagen: | |||
| Yes (n=11; 20%) | 2 (10%) | 9 (25%) | NS |
| No (n=44; 80%) | 17 (90%) | 27 (75%) | |
| Calcifications: | |||
| Yes (n=16; 29%) | 6 (32%) | 10 (28%) | NS |
| No (n=39; 71%) | 13 (68%) | 26 (72%) | |
| C-cell hyperplasia: | |||
| Yes (n=42; 76%) | 8 (42%) | 34 (94%) | 0.02 |
| No (n=13; 24%) | 11 (58%) | 2 (6%) | |
| Multicentricity: | |||
| Yes (n=40; 73%) | 10 (53%) | 30 (83%) | 0.001 |
| No (n=15; 27%) | 9 (47%) | 6 (18%) | |
| Necrosis: | |||
| Yes (n=8; 15%) | 7 (37%) | 1 (3%) | 0.001 |
| No (n=47; 85%) | 12 (63%) | 35 (97%) | |
| Mitosis: | |||
| Yes (n=2; 4%) | 1 (5%) | 1 (3%) | NS |
| No (n=53; 96%) | 18 (95%) | 35 (97%) | |
| Bleeding: | |||
| Yes (n=27; 49%) | 13 (68%) | 14 (39%) | 0.04 |
| No (n=28; 51) | 6 (32%) | 22 (61%) | |
| Vascular invasion: | |||
| Yes (n=22; 40%) | 12 (63%) | 10 (28%) | 0.01 |
| No (n=33; 60%) | 7 (37%) | 26 (72%) | |
| Neovascularization: | |||
| Yes (n=34; 62%) | 15 (79%) | 19 (53%) | 0.04 |
| No (n=21; 38%) | 4 (21%) | 17 (47%) | |
MTC: medullary thyroid carcinoma; NS: not significant.
C-cell hyperplasia was seen in 76% of patients, and multicentricity in 73%. Both characteristics were more common in familial tumors (p<0.001 and p<0.02). However, tumor necrosis was an uncommon finding (15%) mainly associated with sporadic MTC (p<0.001). Mitotic figures were only seen in 2 of the 55 tumors (4%), one each of the familial and sporadic types.
Hemorrhagic foci were found in 49% of tumors, vascular invasion in 40%, and neovascularization in 62%. All three microscopic characteristics were most frequently associated with sporadic MTC (p<0.05), as shown in Table 2.
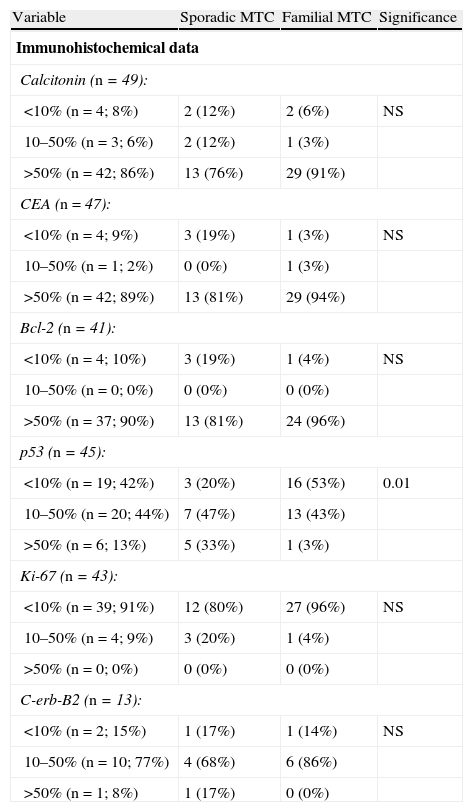
Immunohistochemical characteristicsTumors were strongly positive for calcitonin (86%), CEA (89%), and bcl-2 (90%) markers, with no differences between sporadic and familial tumors, as shown in Table 3.
Differences between sporadic and familial MTC.
| Variable | Sporadic MTC | Familial MTC | Significance |
| Immunohistochemical data | |||
| Calcitonin (n=49): | |||
| <10% (n=4; 8%) | 2 (12%) | 2 (6%) | NS |
| 10–50% (n=3; 6%) | 2 (12%) | 1 (3%) | |
| >50% (n=42; 86%) | 13 (76%) | 29 (91%) | |
| CEA (n=47): | |||
| <10% (n=4; 9%) | 3 (19%) | 1 (3%) | NS |
| 10–50% (n=1; 2%) | 0 (0%) | 1 (3%) | |
| >50% (n=42; 89%) | 13 (81%) | 29 (94%) | |
| Bcl-2 (n=41): | |||
| <10% (n=4; 10%) | 3 (19%) | 1 (4%) | NS |
| 10–50% (n=0; 0%) | 0 (0%) | 0 (0%) | |
| >50% (n=37; 90%) | 13 (81%) | 24 (96%) | |
| p53 (n=45): | |||
| <10% (n=19; 42%) | 3 (20%) | 16 (53%) | 0.01 |
| 10–50% (n=20; 44%) | 7 (47%) | 13 (43%) | |
| >50% (n=6; 13%) | 5 (33%) | 1 (3%) | |
| Ki-67 (n=43): | |||
| <10% (n=39; 91%) | 12 (80%) | 27 (96%) | NS |
| 10–50% (n=4; 9%) | 3 (20%) | 1 (4%) | |
| >50% (n=0; 0%) | 0 (0%) | 0 (0%) | |
| C-erb-B2 (n=13): | |||
| <10% (n=2; 15%) | 1 (17%) | 1 (14%) | NS |
| 10–50% (n=10; 77%) | 4 (68%) | 6 (86%) | |
| >50% (n=1; 8%) | 1 (17%) | 0 (0%) | |
MTC: medullary thyroid carcinoma; NS: not significant.
There were also no differences between the familial and sporadic types in terms of immunoreactivity to Ki-67 (lower than 10% in 91%) or C-erb-B2, in which 77% of tumors showed an intermediate positivity.
44% and 13% of tumors showed intermediate and high positivity for protein p53 respectively, with sporadic tumors more commonly showing a high positivity (p<0.01) (Table 3).
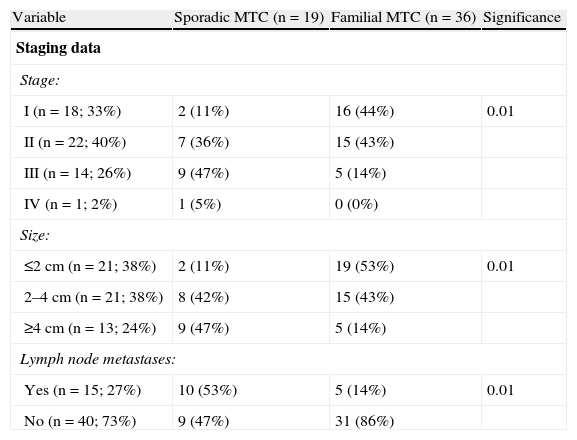
Staging dataTwenty-seven percent of patients (n=14) experienced recurrent MTC. Disease-free rates were 88±5% at 1 year, 73±7% at 5 years, 73±7% at 10 years, 61±10% at 15 years, and 61±10% at 20 or more years. There was a predominance of cases diagnosed and treated at the early disease stages (33% in stage I and 40% in stage II), and there were a few tumors greater than 4cm in diameter (26%) and with lymph node metastases at diagnosis (27%) (Table 4).
Differences between sporadic and familial MTC.
| Variable | Sporadic MTC (n=19) | Familial MTC (n=36) | Significance |
| Staging data | |||
| Stage: | |||
| I (n=18; 33%) | 2 (11%) | 16 (44%) | 0.01 |
| II (n=22; 40%) | 7 (36%) | 15 (43%) | |
| III (n=14; 26%) | 9 (47%) | 5 (14%) | |
| IV (n=1; 2%) | 1 (5%) | 0 (0%) | |
| Size: | |||
| ≤2cm (n=21; 38%) | 2 (11%) | 19 (53%) | 0.01 |
| 2–4cm (n=21; 38%) | 8 (42%) | 15 (43%) | |
| ≥4cm (n=13; 24%) | 9 (47%) | 5 (14%) | |
| Lymph node metastases: | |||
| Yes (n=15; 27%) | 10 (53%) | 5 (14%) | 0.01 |
| No (n=40; 73%) | 9 (47%) | 31 (86%) | |
MTC: medullary thyroid carcinoma; NS: not significant.
Significant differences were found between sporadic and familial MTCs in stage, tumor size, and the presence of nodal metastases. Thus, familial MTC was diagnosed in stage I in 44% of cases, and sporadic MTC in 11% (p<0.01). As regards size, 53% of familial MTCs were 2cm or less in size, as compared to 11% of sporadic MTCs (p<0.01). Finally, no nodal metastases were found in 86% of familial MTCs, as compared to 47% of sporadic MTCs (p<0.01).
DiscussionThe epidemiological characteristics of MTC vary depending on the geographic area. Thus, a high prevalence of familial tumors (66%), four times higher than the expected mean,20,21 was found in our series. The reason for this is that there is a high incidence of MEN syndrome in our geographical area.17,22 This high incidence of familial tumors is the reason for some of the differences found as compared to other studies such as, for instance, in mean age. This was because many patients were diagnosed in screening programs in carrier families, which allow for discovering the disease at very early stages.17
As regards the microscopic characteristics of MTC, there is no agreement between authors in classification of the different cytoarchitectural patterns. Thus, Franc et al.11 consider 8 architectural and 9 cellular characteristics, while others such as Dottorini et al.12 or Schröder et al.13 only consider three cytological types, and Kos et al.14 three architectural types only. Despite this lack of agreement, cells of a plasmacytoid or polygonal morphology12,13 arranged in a solid growth pattern9,13,14,23 predominate in most series. A predominant fusocellular cytological type was only found in 4% of tumors in our series, while in other series this type was reported in more than 20% of tumors.13 It should be noted, however, that while we only considered the predominant characteristic, other authors such as Franc et al.11 gave more weight to differential features, which may partly account for the differences between the series.
The histological appearance of familial and sporadic MTC was similar, except for C-cell hyperplasia and multicentricity, which were more common in familial tumors. However, cases of sporadic MTC with C-cell hyperplasia are common (42%), and we also found a few cases of familial MTC (6%) where C-cell hyperplasia could not be shown. Both findings had previously been reported, and it has been postulated that C-cell hyperplasia is also the precursor of sporadic MTC.24 Thus, the Kebebew et al. study reported bilateral involvement in 67% of sporadic tumors20 and suggested that up to 10% of sporadic MTCs may be familial tumors that had been overlooked. This may have occurred with the oldest cases in our series. Other authors such as Hayashida et al. suggest that the lack of C-cell hyperplasia in familial cases may be due to inadequate sampling of areas with no tumor in thyroidectomy specimens.25
Amyloid deposits, considered a typical characteristic of MTC, could only be found in 73% of tumors, a similar proportion to that reported by Bergholm et al.15 and Schröder et al.13 but much lower than found in other series.12,23 On the other hand, studies rarely report the presence of calcifications, which were found in 29% of the patients in our study, a high percentage.13
As regards all other microscopic characteristics, the presence of necrotic areas greatly varies depending on the series, ranging from 50%11 to 7%.12 An intermediate figure, 15%, was found in our study. Mitotic activity is usually uncommon (4% in our series), and while some authors expressly mention it,23 no specific data are usually given.
The histological characteristics commonly associated with greater tumor aggressiveness, such as necrosis, bleeding, vascular invasion, and neovascularization, are more frequently associated with sporadic MTC. This association is very likely to be due rather to tumor size, because sporadic MTC is diagnosed and resected when it has a greater size as compared to familial MTC.
All these data led us to state that there are no histopathological criteria allowing for differentiation of the familial and sporadic forms of MTC.
Controversy also exists about immunohistochemical characteristics, as not all authors agree as to how the degree of positivity should be quantitated,9,13,15 and changes also exist in the sensitivity of the different antisera used.9,13 These tumors usually show a strong positivity for calcitonin,26 CEA,12 and bcl-227,28 (found in 86%, 89%, and 90% of tumors in our series, respectively). Other studies using different methods have reported different results. Thus, Bergholm et al.15 found positive results for calcitonin in 26% of tumors, Kos et al.14 in 11%, etc. Conflicting immunohistochemical findings may suggest a potential degradation of the target antigen in the paraffin block due to long-term or inadequate material storage.9
Results for other markers are less well known and heterogeneous. Thus, very low positive rates have been reported for p53 (5–28%).27,28 However, we found more than 13% of tumors with more than 50% of cells stained for p53 and 44% with 10%-50% stained cells. It has been reported that approximately 40% of malignant thyroid tumors are positive for Ki-67,29 but the positivity rate found in our series, focusing on MTC only, was much lower. Thus, this antigen was poorly expressed or absent in 91% of tumors. Information about C-erb-B2 and thyroid gland is very scanty and does not refer to MTC. Thus, Soda et al.30 reported that 60% of malignant thyroid tumors were positive for C-erb-B2. We found an intermediate or high positivity in almost 85% of tumors.
As regards the immunohistochemical characteristics, and specifically immunoreactivity, the only difference seen between the familial and sporadic varieties was a more frequent low positivity (less than 10% of cells stained) for p53 in familial MTC and a high positivity (more than 50% of cells stained) in sporadic MTC, which may be related to the presence of aggressive forms within the sporadic group. Immunohistochemistry is not helpful for discriminating between sporadic and familial cases of MTC.31
Potential immunohistochemical markers that may possibly have some prognostic or diagnostic significance for these tumors in the future are appearing. Thus, the expression in MTC of different proteins such as protein p8, which appears to have a significant role in MTC progression, has been reported.32 Ito et al.33 analyzed expression of cdc25B and cdc25A in MTC and found that cdc25B is a marker of aggressiveness and is expressed in 36% of tumors.
Finally, as regards staging and clinical course, a great majority of our patients underwent surgery at early stages of the disease because there were several familial cases diagnosed by genetic study. Hence, while a majority of studies reported nodal metastases and thus stage III or IV disease (>40%),15,21,23,34,35 we only found them in 28% in stage III and in 2% in stage IV. Most patients diagnosed with stage I tumors, i.e. less than 1cm in size and with no metastases, therefore have familial MTC, while most patients undergoing surgery for stage III disease, greater than 4cm in diameter or with nodal metastases, usually have sporadic MTC. This earlier detection at a younger age and at earlier stages accounts for the better prognosis of familial forms, according to Goudet et al. and Samaan et al.36,37
In conclusion, MTCs have a solid growth pattern and plasmacytoid cells, with frequent presence of amyloid, C-cell hyperplasia, multicentricity, and neovascularization. Immunoreactivity is high for calcitonin, CEA, and bcl-2, low for Ki-67, and intermediate for p53 and C-erb-B2. A comparison of sporadic and familial cases shows that C-cell hyperplasia and multicentricity more commonly occur in familial MTC, but are not exclusive to this tumor, while necrotic foci, bleeding, vascular invasion, and neovascularization are more common in sporadic MTC. Neither histopathological nor immunohistochemical criteria are helpful in differentiating the familial and sporadic forms of MTC.
Conflicts of interestThe authors have no conflict of interest to declare.
Please, cite this article as: Ríos A, et al. Perfil histológico e inmuno-histoquímico del carcinoma medular de tiroides esporádico y familiar. Endocrinol Nutr. 2011;58:521–8.