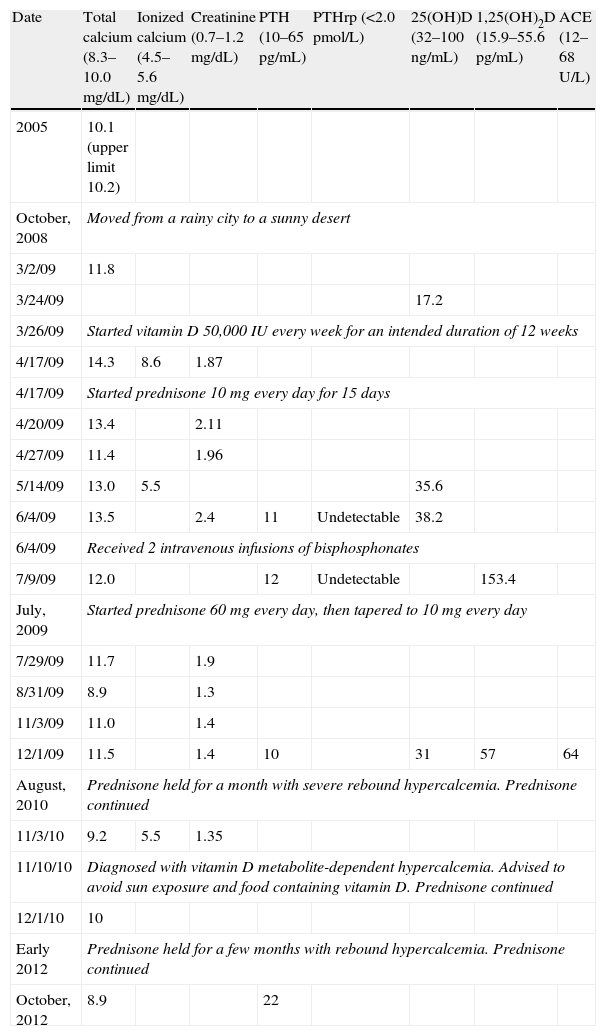
I wish to describe a patient with vitamin D metabolite-dependent hypercalcemia which only manifested after he moved from a rainy city to a sunny desert. This 73-year-old man began to feel vaguely unwell after he had moved from a city in Washington State (with cloudy and rainy weather) to a desert area in California State (with sunny weather in >300days/year), USA, in October, 2008. His past medical history was significant for hypothyroidism and mild anemia. His calcium levels had been at the upper end of normal in 2005 (Table 1) and small stones found in bladder during a transurethral prostate resection in 2007. He did not take vitamin supplements; he had been mostly indoors before but played golf frequently after the move. In March, 2009, his new physician noted mild hypercalcemia and vitamin D deficiency and gave him high-dose vitamin D (Table 1). Three weeks later, severe hypercalcemia (14.3mg/dL) was found on follow-up testing, which was attributed to possible occult cancer; CT scan of the chest, abdomen, and pelvis, however, did not reveal any tumors. The patient was given intravenous hydration and prednisone with improvement of hypercalcemia. Five weeks later, hypercalcemia recurred; parathyroid hormone (PTH) levels were suppressed, PTH-related peptide (PTHrp) undetectable, and 25-hydroxyvitamin D at lower end of normal. He received intravenous bisphosphonates. Markedly elevated 1,25-dihydroxyvitamin D levels (153.4pg/mL) were found a month later (Table 1). Lymphoma, multiple myeloma, and granulomatous diseases were suspected. Repeat CT scan revealed mediastinal (a 1.8×2-cm precarinal and a 1.6×1.7-cm aortopulmonary window lymph node) and celiac (a 1.4×1.5-cm lymph node) lymphoadenopathy, and bone scan and bone marrow biopsy returned normal findings. The precarinal lymph node was resected in July, 2009, but hypercalcemia persisted. The lymph node showed reactive features on histological examination. 1,25-Dihydroxyvitamin D levels were normalized after prednisone treatment which could not be discontinued without severe hypercalcemia (Table 1).
Timeline of calcium and calcium-regulating hormone levels. PTH, parathyroid hormone; PTHrp, PTH-related peptide; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; and ACE, angiotensin converting enzyme.
| Date | Total calcium (8.3–10.0mg/dL) | Ionized calcium (4.5–5.6mg/dL) | Creatinine (0.7–1.2mg/dL) | PTH (10–65pg/mL) | PTHrp (<2.0pmol/L) | 25(OH)D (32–100ng/mL) | 1,25(OH)2D (15.9–55.6pg/mL) | ACE (12–68U/L) |
| 2005 | 10.1 (upper limit 10.2) | |||||||
| October, 2008 | Moved from a rainy city to a sunny desert | |||||||
| 3/2/09 | 11.8 | |||||||
| 3/24/09 | 17.2 | |||||||
| 3/26/09 | Started vitamin D 50,000IU every week for an intended duration of 12 weeks | |||||||
| 4/17/09 | 14.3 | 8.6 | 1.87 | |||||
| 4/17/09 | Started prednisone 10mg every day for 15 days | |||||||
| 4/20/09 | 13.4 | 2.11 | ||||||
| 4/27/09 | 11.4 | 1.96 | ||||||
| 5/14/09 | 13.0 | 5.5 | 35.6 | |||||
| 6/4/09 | 13.5 | 2.4 | 11 | Undetectable | 38.2 | |||
| 6/4/09 | Received 2 intravenous infusions of bisphosphonates | |||||||
| 7/9/09 | 12.0 | 12 | Undetectable | 153.4 | ||||
| July, 2009 | Started prednisone 60mg every day, then tapered to 10mg every day | |||||||
| 7/29/09 | 11.7 | 1.9 | ||||||
| 8/31/09 | 8.9 | 1.3 | ||||||
| 11/3/09 | 11.0 | 1.4 | ||||||
| 12/1/09 | 11.5 | 1.4 | 10 | 31 | 57 | 64 | ||
| August, 2010 | Prednisone held for a month with severe rebound hypercalcemia. Prednisone continued | |||||||
| 11/3/10 | 9.2 | 5.5 | 1.35 | |||||
| 11/10/10 | Diagnosed with vitamin D metabolite-dependent hypercalcemia. Advised to avoid sun exposure and food containing vitamin D. Prednisone continued | |||||||
| 12/1/10 | 10 | |||||||
| Early 2012 | Prednisone held for a few months with rebound hypercalcemia. Prednisone continued | |||||||
| October, 2012 | 8.9 | 22 | ||||||
The patient came to our institution for definitive diagnosis of the cause of hypercalcemia in November, 2010. He took prednisone 10mg daily and his calcium levels normal. He felt well in general. As the patient had suppressed PTH, undetectable PTHrP, but markedly elevated 1,25-dihydroxyvitamin D levels, he was diagnosed with vitamin D metabolite-dependent hypercalcemia, and was advised to continue prednisone and to avoid sun exposure and to decrease intake of food items containing vitamin D. The patient found it hard to avoid sun exposure in his new home area where it is sunny in >300days/year because he enjoyed the numerous outdoor activities there. He continued to require prednisone 10mg daily with normocalcemia and without developing Cushingoid features. The source of the ectopic 1-hydroxylase activity remained unclear but pulmonary sarcoidosis was considered a possibility.
Few endocrine diseases are affected by geographic factors. Although there could be multiple potential causes of the patient's clinical course and it is possible that he acquired ectopic 1-hydroxylase activity upon moving to the sunny desert, the most parsimonious explanation of the patient's clinical course is long-standing ectopic 1-hydroxylase activity.1 When he lived in Washington State, he was probably severely vitamin D-deficient due to the cloudy and rainy weather and lack of vitamin supplements so that the ectopic 1-hydroxylase did not have enough substrate to make large amounts of 1,25-dihydroxyvitamin D, the active metabolite. He presumably had mildly elevated 1,25-dihydroxyvitamin D levels, causing “high normal” calcium levels and urolithiasis. After he moved to California, the sun exposure likely resulted in an increase of 25-hydroxyvitamin D levels which allowed the ectopic 1-hydroxylase to make enough 1,25-dihydroxyvitamin D to cause mild hypercalcemia, as shown previously in patients with sarcoidosis or other conditions.2,3 The worsening of hypercalcemia by large-dose vitamin D replacement and the improvement of hypercalcemia by corticosteroids both support the diagnosis, although the former could have been avoided by searching the cause of the mild hypercalcima systemically rather than viewing 25-hydroxyvitamin D levels in isolation. Vitamin D metabolite-dependent hypercalcemia is a rare syndrome; its causes are many, such as sarcoidosis, lymphoma, and leukemia, and may remain unknown for years, as in this patient1. This case thus highlights the geographic influence on the presentation of endocrine diseases and calls for attention on interpreting laboratory test results in the context of personal history.
Conflicts of interestThe author has no conflicts of interest to disclose.