Glucagon-like peptide 1 (GLP-1) agonists decrease glucose levels with a very low risk of hypoglycemia, limit weight increase associated to insulin therapy and have favorable effects on dyslipidemia, high blood pressure, endothelial function, cardiac contractility, intestinal lipoproteins, inflammation, and indirect kidney function markers.1–3
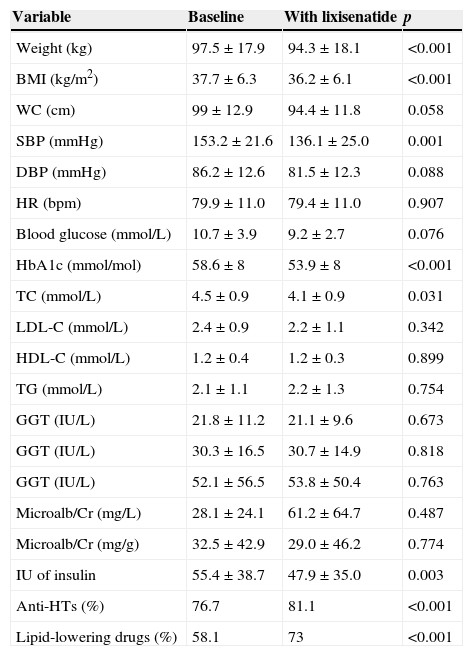
A prospective study with within-subject measures of change was conducted on 43 patients (62.8% females) with type 2 diabetes mellitus and obesity who started treatment with lixisenatide (Lyxumia®, Sanofi Aventis S.A.) to assess drug tolerability and impact on weight and metabolic control. Subgroups without and with prior antihypertensive treatment (23.3% and 76.7% respectively) and lipid-lowering treatment (41.9% and 58.1% respectively) and occurrence of side effects were analyzed. Mean age was 58±13.4 years, and mean time since diabetes onset 11.9±7.3 years. A family history of cardiovascular disease (CVD) and diabetes was found in 41.4% and 62.9% of patients respectively. At the baseline visit, treatment consisted of oral agents (86% of patients), GLP-1 analogues (18.6%), and insulin (79.1%; basal 37.2%, premixes 25.6%, and basal-bolus 16.3%). Mean follow-up time from start of lixisenatide was 3.9±1.5 months. Table 1 shows change over time in clinical and laboratory variables. Improvement in systolic blood pressure (SBP) and total cholesterol (TC) was significant in the subgroups without and with antihypertensives before and after treatment with lixisenatide (SBP before 147±26.5mmHg vs SBP after 113.6±7.9mmHg, p=0.023; and SBP before 153.6±21mmHg vs SBP after 140.0±25.4mmHg, p=0.020, respectively), while TC decrease only remained significant in the subgroup taking lipid-lowering drugs before lixisenatide was started (TC before 178.5±36mg/dL vs TC after 152.6±25.6mg/dL; p=0.007), in which a significant decrease was also seen in LDL cholesterol (LDL-C) (LDL-C before 93.3±37.2mg/dL vs LDL-C after 73.1±25.9mg/dL; p=0.023). No change was seen in amylase levels (mean amylase level 40.6±6.7IU/L). As regards digestive tolerability, 20% did not tolerate the drug, 5% tolerated 10 mcg/day, and 75% tolerated 20 mcg/day.
Change in clinical and laboratory variables before and after lixisenatide treatment.
| Variable | Baseline | With lixisenatide | p |
|---|---|---|---|
| Weight (kg) | 97.5±17.9 | 94.3±18.1 | <0.001 |
| BMI (kg/m2) | 37.7±6.3 | 36.2±6.1 | <0.001 |
| WC (cm) | 99±12.9 | 94.4±11.8 | 0.058 |
| SBP (mmHg) | 153.2±21.6 | 136.1±25.0 | 0.001 |
| DBP (mmHg) | 86.2±12.6 | 81.5±12.3 | 0.088 |
| HR (bpm) | 79.9±11.0 | 79.4±11.0 | 0.907 |
| Blood glucose (mmol/L) | 10.7±3.9 | 9.2±2.7 | 0.076 |
| HbA1c (mmol/mol) | 58.6±8 | 53.9±8 | <0.001 |
| TC (mmol/L) | 4.5±0.9 | 4.1±0.9 | 0.031 |
| LDL-C (mmol/L) | 2.4±0.9 | 2.2±1.1 | 0.342 |
| HDL-C (mmol/L) | 1.2±0.4 | 1.2±0.3 | 0.899 |
| TG (mmol/L) | 2.1±1.1 | 2.2±1.3 | 0.754 |
| GGT (IU/L) | 21.8±11.2 | 21.1±9.6 | 0.673 |
| GGT (IU/L) | 30.3±16.5 | 30.7±14.9 | 0.818 |
| GGT (IU/L) | 52.1±56.5 | 53.8±50.4 | 0.763 |
| Microalb/Cr (mg/L) | 28.1±24.1 | 61.2±64.7 | 0.487 |
| Microalb/Cr (mg/g) | 32.5±42.9 | 29.0±46.2 | 0.774 |
| IU of insulin | 55.4±38.7 | 47.9±35.0 | 0.003 |
| Anti-HTs (%) | 76.7 | 81.1 | <0.001 |
| Lipid-lowering drugs (%) | 58.1 | 73 | <0.001 |
Data are given as mean±standard deviation.
WC: waist circumference; TC: total cholesterol; HR: heart rate; GGT: gamma glutamyltranspeptidase; GOT: glutamic oxaloacetic transaminase; GPT: glutamic pyruvic transaminase; HbA1c: glycosylated hemoglobin; HDL-C: HDL cholesterol; HBP: high blood pressure; BMI: body mass index; LDL-C: LDL cholesterol; Microalb/Cr: microalbumin/creatinine ratio; DBP: diastolic blood pressure; SBP: systolic blood pressure; TG: triglycerides; IU: insulin units.
No significant improvement in fasting blood glucose was seen in our study. GLP-1 analogues have been shown to achieve similar improvements in basal blood glucose, but lixisenatide 20 mcg achieves greater reductions in the area under the curve as compared to postprandial blood glucose and greater slowing of gastric emptying.4 A significant decrease in HbA1c was seen in our patients. GLP-1 analogues have shown to be non inferior to other hypoglycemic agents5 and to achieve greater and similar HbA1c reductions in a meta-analysis as compared to oral agents and basal and biphasic insulin respectively.6
Significant weight and BMI decreases were seen, consistent with reports by other authors,7 with a mean weight loss of 3.2kg. These results are similar to those previously reported of decreases by 1.4kg and 4.8kg versus placebo and insulin respectively,5 as well as reductions by 3.31kg versus diabetic controls on other treatments and by 1.22kg versus placebo in a meta-analysis.8
Despite similar HbA1c decrease, 35% less hypoglycemic episodes were reported with GLP-1 as compared to insulin.7 Meier et al. found greater risk of hypoglycemia with lixisenatide as compared to liraglutide,4 while Rosenstock et al. reported less episodes of symptomatic hypoglycemia in the lixisenatide group (2.5% vs 7.9%, p<0.05).9 In agreement with this last study, our patients had no symptomatic hypoglycemia.
Lixisenatide has been reported to have a more favorable profile of digestive tolerability than exenatide twice daily9 and liraglutide.4 Most patients in our study tolerated well lixisenatide treatment.
As regards lipid profile, improvement was seen in TC levels and remained significant in the subgroup already taking lipid-lowering drugs. A significant LDL-C decrease was also found. In agreement with our results, other authors have reported lipid improvement in patients treated with GLP-1 analogues.1–3
Treatment with lixisenatide decreased SBP values, with no statistical significance achieved as compared to DBP levels. These results agree with reports by other authors.7,8
No significant changes were found in heart rate (HR). However, a meta-analysis concluded that GLP-1 analogues increase HR by 1.86 beats per minute versus placebo and by 1.90 beats per minute as compared to other antidiabetics, an effect which was more evident with liraglutide as compared to exenatide twice daily.8 Lixisenatide causes a lower increase5 and even a decrease in HR10 as compared to liraglutide.
Limitations of our study include the small number of studies reported in standard clinical practice with which our results could be compared, the small sample size, the short follow-up time, and lack of assessment of postprandial blood glucose.
Our analysis shows that treatment with lixisenatide significantly improves anthropometric parameters, and blood glucose control in terms of HbA1c with less insulin requirements, and causes significant SBP and TC decreases. Tolerability was good in most patients. A significant intensification of antihypertensive and lipid-lowering treatment, not only hypoglycemic therapy, was also seen in our clinical practice, with an overall metabolic approach to patients. Longer studies with larger sample sizes are needed to assess the long-term efficacy and safety of therapies with GLP-1 analogues.
FundingA grant given by the Scientific Committee of the 26th National Congress of the Spanish Diabetes Society thanks to the cooperation of AstraZeneca.
Conflict of interestThe authors state that they have no conflicts of interest.
To patients participating in the study, the Spanish Diabetes Society, and Dr. Gabriel Olveira-Fuster, head of the UGC of Endocrinology and Nutrition, Hospital Regional Universitario Carlos Haya, Málaga.
Please cite this article as: Roca-Rodríguez MM, Tapia-Guerrero MJ, Maraver-Selfa S, Tinahones FJ, Mancha-Doblas I. Experiencia clínica con lixisenatida en pacientes con diabetes tipo 2 y obesidad en consultas de atención especializada en Málaga. Endocrinol Nutr. 2015;62:512–514.