Sensor-augmented pump (SAP) therapy has been shown to be effective and safe for improving metabolic control in patients with type 1 diabetes mellitus (T1DM) in a number of trials. Our objective was to assess glycemic control in a group of T1DM patients on insulin pump or SAP therapy after years of participating in the SWITCH (Sensing With Insulin pump Therapy To Control HbA1c) trial and their return to routine medical monitoring.
MethodsA retrospective, observational study of 20 patients who participated in the SWITCH trial at our hospital from 2008 to 2010. HbA1c values were compared at the start, during (at the end of the periods with/without SAP use – Sensor On/Sensor Off period respectively – of the cross-over design), and 3 years after study completion. HbA1c values of patients who continued SAP therapy (n=6) or only used insulin pump (n=14) were also compared.
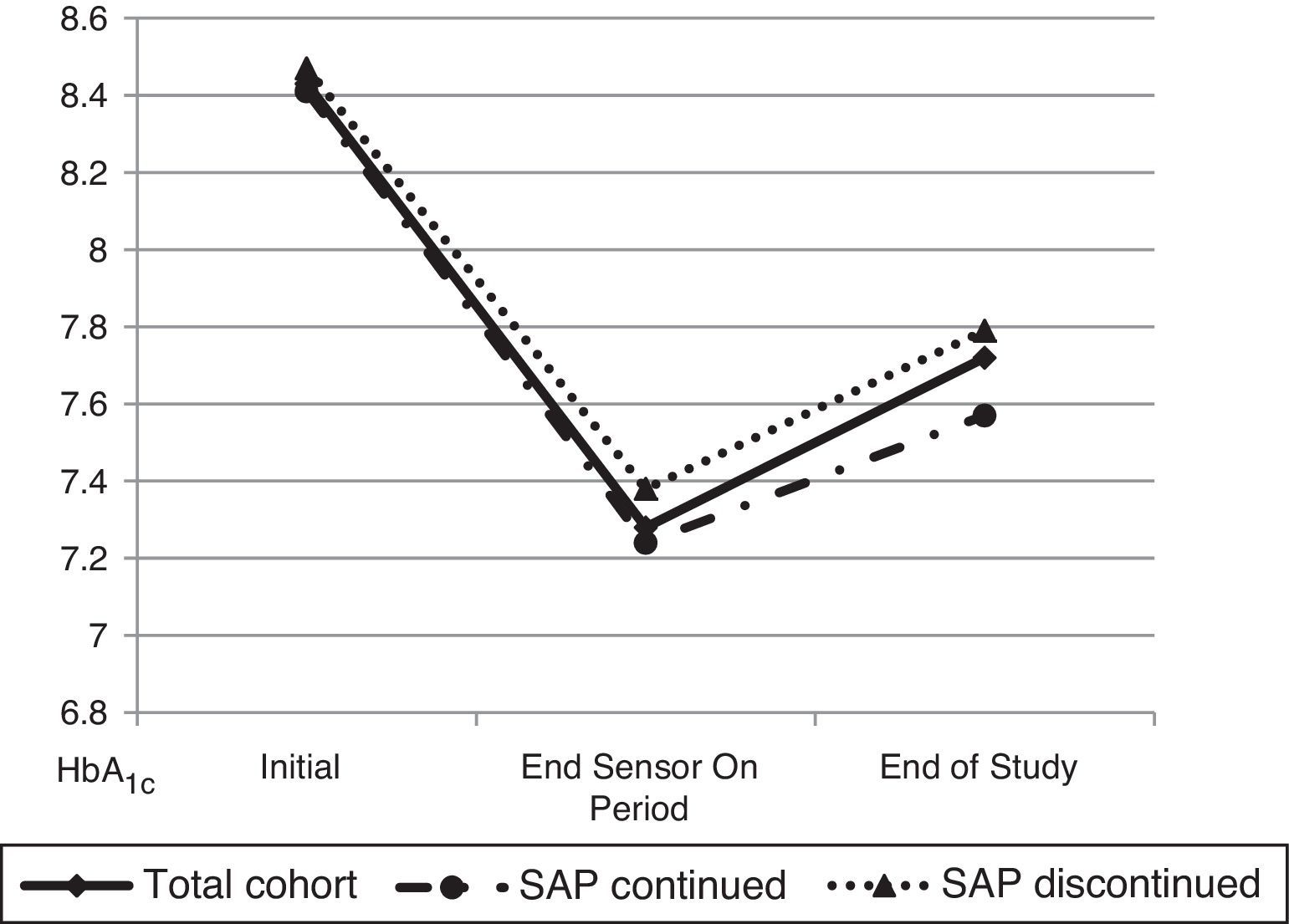
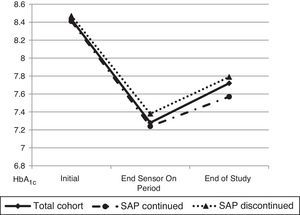
ResultsTwenty patients with T1DM (44.4±9.3 years, 60% women, baseline HbA1c level 8.43±0.55%) were enrolled into the SWITCH study). Three years after study completion, HbA1c level was 7.79±0.77 in patients on pump alone, with no significant change from the value at the end of the Off period of the study (7.85±0.57%; p=0.961). As compared to the end of the On period, HbA1c worsened less in patients who remained on SAP than in those on pump alone (0.18±0.42 vs. 0.55±0.71%; p=0.171), despite the fact that levels were similar at study start (8.41±0.60 vs. 8.47±0.45; p=0.831) and at the end of the On period (7.24±0.48 vs. 7.38±0.61; p=0.566). Frequency of CGM use in patients who continued SAP therapy was high (61.2% of the time in the last 3 months).
ConclusionsOur study suggests that the additional benefit of SAP therapy achieved in a clinical trial may persist in the long term in routine clinical care of patients with T1DM.
La terapia bomba-sensor (SAP, del inglés Sensor Augmented Pump) ha demostrado eficacia y seguridad en la mejoría del control metabólico en pacientes con diabetes tipo 1 (DT1) en múltiples ensayos clínicos. Nuestro objetivo ha sido valorar el control glicémico en un grupo de pacientes con DT1 en tratamiento con bomba de insulina/SAP años después de su participación en el estudio SWITCH (Sensing With Insulin Pump Therapy To Control HbA1c) tras el retorno al seguimiento médico habitual.
MétodosEstudio observacional retrospectivo que incluye todos los pacientes que participaron en el estudio SWITCH en nuestro centro entre 2008 y 2010. Se comparó la HbA1c al inicio, durante (al final de los periodos con/sin terapia SAP – periodos Sensor On/Sensor Off respectivamente del diseño cruzado-) y tres años tras la conclusión del estudio. Adicionalmente, se compararon los valores de HbA1c de los pacientes que habían continuado la terapia SAP (n=6) respecto a los que únicamente utilizaban bomba de insulina (n=14).
ResultadosSe incluyeron 20 pacientes con DT1 (44.4±9.3 años, 60% mujeres, HbA1c al inicio del estudio SWITCH 8.43±0.55%). Tres años después de la conclusión del estudio, la HbA1c en los pacientes que únicamente realizaban tratamiento con bomba fue de 7.79±0.77%, sin cambios significativos desde la finalización del periodo Off del estudio (7.85±0,57%, p=0.961). En comparación con la conclusión del periodo On, la HbA1c de aquellos pacientes que mantuvieron la terapia SAP al finalizar el estudio empeoró menos que aquellos que únicamente utilizaban bomba (0.18±0.42 vs. 0.55±0.71%; p=0.171) aun siendo igual tanto al inicio del estudio (8.41±0.60 vs. 8.47±0.45; p=0.831) como al finalizar el periodo On (7.24±0.48 vs. 7.38±0.61; p=0.566). Los pacientes que seguían realizando terapia SAP tenían un elevado uso del sensor (61.2% del tiempo durante los últimos 3 meses).
Conclusionesnuestro estudio apunta que el beneficio adicional obtenido por la terapia SAP durante un ensayo clínico puede persistir a largo plazo durante la práctica clínica habitual en los pacientes con DT1.
Long-term benefits of tight glycemic control in patients with type 1 diabetes (T1D) have been widely demonstrated through various studies derived from Diabetes Control and Complications Trial (DCCT).1,2 However, despite the development of insulin analogs and continuous subcutaneous insulin infusion therapy (CSII), achieving this tight glycemic control continues to be a challenge in a number of individuals with T1D.
In recent years, the introduction of real-time continuous glucose monitoring (CGM) devices associated with both multiple doses of insulin (MDI) therapy or CSII (sensor augmented pump – SAP), has shown an improvement in glycemic control without increasing the rate of hypoglycaemia in some clinical trials.3,4 However, this improvement is strictly associated with the frequency of use of the device, which explains why in other clinical trials where the time of use of the device was low (in the total cohort or in part of that one) these benefits have not been shown.5,6
As in all evaluations with technological products, the results of controlled clinical trials not only reflect the tested technology but also other variables associated with the development of clinical trials such as frequent visits, educative intervention associated or highly qualified healthcare teams. Therefore, it is important to confirm that these results are obtained in usual care. In order to verify this, many observational studies with divergent results have been published.7,8
Consequently, we have conducted a retrospective observational study in order to assess the degree of glycemic control of a group of T1D patients treated with CSII with/without CGM during usual care 3 years after completing their participation in a clinical trial about CGM (SWITCH study – Sensing With Insulin pump Therapy To Control HbA1c).9
MethodsRetrospective observational study that included the 20 patients with T1D in treatment with insulin pump that had participated in SWITCH study in the Diabetes Unit, Hospital Clinic i Universitari of Barcelona from 2008 to 2010. This study was a cross-over randomized clinical trial where patients were assigned to two 6 month periods during which they were treated with CSII only (Off period) or SAP therapy (On period). Inclusion criteria and design of the study had been previously published by Conget et al.10
Demographic data were collected at the beginning of the SWITCH study as well as the HbA1c at the beginning of the study, at the end of each period (On/Off) and at the end of the study. After that, data on the therapy that each patient had continued after the end of the study and data regarding the degree of metabolic control 3 years after the end of the trial during usual care in our unit were collected. Moreover, data on severe hypoglycaemia episodes in the last year were collected from clinical records and data on non-severe hypoglycemia frequency (capillary or sensor glucose <70mg/dL) in the last month were analyzed from the glucose meter/sensor download into the Carelink PRO software (Medtronic, Northridge, CA, US). Finally, a comparative analysis between patients who continue SAP therapy and patients who only use CSII was performed.
Data are shown as mean and standard deviation. Mean comparison was done by t-Student test for non-paired data for normal variables and by U-Mann–Whitney for non-normal variable. A p-value≤0.05 was considered statistically significant. The analysis was performed using SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, EE, UU).
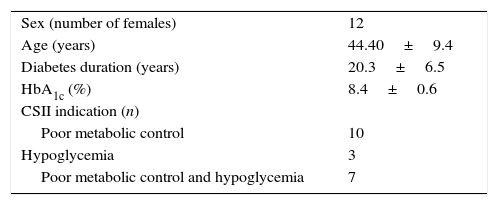
ResultsData from all 20 patients previously included in the SWITCH study were obtained. 60% (12) were women, mean age at the inclusion of the study was 44.4±9.3 years, with a diabetes duration of 20.3±6.5 years, and mean HbA1c of 8.43±0.55%. In 50% of the patients the reason to start CSII therapy was suboptimal metabolic control, in 15% was the presence of frequents and disabling hypoglycaemic events and in the remaining 35% was the combination of both (Table 1).
The mean HbA1c at the end of three years of follow up was 7.72±0.67% while at the end of the On period of the SWITCH study (period in which patients used SAP) was 7.28±0.51% and at the end of the Off period was (7.85±0.57%, p=0.961).
Later, a comparative analysis between patients that had continued SAP therapy 3 years after (6 patients, 30%) and patients that had returned to CSII therapy at the end of the SWITCH study was performed (Fig. 1). At the beginning of the trial both groups of patients had similar HbA1c (8.41±0.60 vs. 8.47±0.45; p=0.831). Likewise, HbA1c at the end of the On period was not different between the two groups (7.24±0.48 vs. 7.38±0.61; p=0.566). 3 years after the end of the study, patients who continued SAP therapy had a mean HbA1c of 7.57±0.47% while patients who had stopped SAP therapy (remain only in pump) had an HbA1c of 7.79±0.77% (p=0.50).
Regarding hypoglycaemia, there were no episodes of severe hypoglycemia in the last year of follow up. In the total cohort the number of non-severe hypoglycaemia episodes/week was 1.97±1.78.
Furthermore, the degree of worsening in metabolic control from the end of the On period to 3 years after the end of the study was evaluated. The result was 0.18 points rise of HbA1c in patients who continued SAP therapy in front of 0.55 points in patients who discontinued SAP (p=0.171). The frequency of CGM use in patients who continued SAP therapy was 61.2% of the time in the prior 3 months before the last HbA1c determination.
Finally, data between the beginning of the study and 3 years after it ended were compared in order to evaluate the possible influence of the educational component derived from the participation in a trial with these characteristics. In the global cohort, HbA1c changed from 8.43±0.55% at the beginning of the trial to 7.72±0.69% 3 years after conclusion (p=0.004). Considering only patients who had not maintained SAP after the end of the study, these values were 8.39±0.62 and 7.78±0.77% respectively (p=0.062).
DiscussionThis study shows that the benefit achieved by SAP therapy during a controlled trial may persist long-term during usual care. The improvement obtained by patients who participated in the SWITCH study in the Diabetes Unit of Hospital Clinic during the period which they used CGM, was maintained 3 years after the end of the study (worsening of 0.18 points of HbA1c) in patients who continued SAP therapy. However, patients who discontinued this therapy and returned to CSII (without CGM) worsened 0.55 points, in spite of similar metabolic control before starting the SWITCH study.
In 2012 the SWITCH study10 showed additional benefits of SAP therapy in comparison with pump therapy alone in suboptimal controlled patients with type 1 diabetes. These benefits had been shown previously by Bergenstal et al.3 comparing intensive treatment with multiple doses of insulin and by Deiis et al.11 comparing both treatment modalities. In each case, these benefits were obtained without increasing time on hypoglycemic range. However, other studies have been published with contradictory findings. For instance, the study published by Raccah et al.12 in 2009 obtained a significant improvement in HbA1c in the analysis of patients that had used CGM at least 70% of the time, but not in the analysis of the total cohort. Moreover, the study published by JDRF in 20086 only showed improvement in metabolic control in the adults that used CGM 83% of time, but not in children or adolescents that used it 50% and 30% of the time respectively. Other studies that failed to demonstrate an improvement in HbA1c5 showed that the level of adherence to CGM was directly related with glycemic control improvement in a secondary analysis.
As a result of having evidence that in controlled clinical trials SAP therapy obtain benefits in patients that use it most of the time, other studies have been designed to assess its efficacy in usual care. In 2013 the INTERPRET study7 including 263 patients from different countries was published. This study did not show improvement in HbA1c after 12 months of SAP therapy use during usual care. However, the use of CGM was less than 30% of the time in the total cohort and it was demonstrated again that the level of sensor use was significantly correlated with the improvement in HbA1c.
In our study, the use of sensor was quite high (61.2% of time in the last 3 months), which could explain why metabolic control did not worsen after the clinical trial. Considering that in our environment SAP therapy is not funded by the public health system, it is easy to understand that patients who decided to continue this therapy after the results obtained during the trial have an elevated use of sensor in long-term. Moreover, the patients included in this study are only adults which, as seen in some previous publications,6 are more adherent to this therapy than other age groups.
The main limitation of the study is the small number of patients, which limits the possibility to find statistically significant differences although, in our opinion, observed differences are clinically relevant. Furthermore, it is an observational retrospective study, with its limitations, but probably this sort of studies contributes adequately to evaluate any technology in usual care. Randomized clinical trials and real-life studies both have limitations and should be seen as complementary. Finally, we recorded the non-severe hypoglycaemia episodes downloading data in Carelink PRO software from glucose meter in patients who did not continue SAP therapy and glucose meter and sensor in patients who continued this therapy. We are aware that sensor data detects more non-severe hypoglycaemia episodes than glucose meter data download, for this reason we were unable to compare the frequency of minor hypoglycaemia episodes in both groups.
As a conclusion, this study indicates that the additional benefit of SAP therapy obtained in a clinical trial may persist long-term in the routine medical care of patients with T1D. However larger studies are necessary to confirm this point in order to convince authorities that SAP is an efficient therapy for some patients with type 1 diabetes.
Conflict of interestsThe authors declare no conflict of interest.