Prevalence of hyperandrogenism (HA), including the polycystic ovary syndrome (PCOS), in female-to-male transsexuals (FMT) is high. This has been related to metabolic syndrome (MS), which appears to increase cardiovascular morbidity and mortality throughout cross-sex hormone (CSH) therapy.
ObjectivesTo assess the prevalence of HA and PCOS in FMT patients before the start of CSH therapy, and their association to MS and its components, insulin resistance (IR) and other cardiovascular risk (CVR) factors.
Materials and methodsSeventy-seven FMTs underwent clinical and biochemical assessment for HA before the start of CSH therapy. CVR, IR, and other MS parameters were also assessed.
ResultsPrevalence of HA was 49.4% (73.7% were cases of PCOS [Rotterdam criteria]), and prevalence of PCOS in the overall sample was 36.4%. Prevalence of MS was 38.4% and 51.7% according to ATP-III and IDF criteria respectively). MS (according to ATP-III and IDF criteria respectively) was found in 36.8% and 57.9% as compared to 25.6% and 41% of patients with and without HA respectively (p<0.0001 and p<0.01 respectively). Of total patients, 54.5% had normal weight (body mass index [BMI] 18.5–24.9kgm−2), 26% were overweight (BMI 25–29.9kgm−2), and 19.5% were obese (BMI≥30kgm−2). After adjusting for BMI, the comparison of hormonal, metabolic, and anthropometric parameters showed statistically significant differences in plasma glucose, HOMA-IR, and abdominal circumference (p<0.001 for all), as well as HDL cholesterol (HDL) (p=0.033), but not in total testosterone or calculated free testosterone levels. In the total sample, 27.3% had HDL levels less than 50mg/dL.
ConclusionsOverall HA, and PCOS in particular, are highly prevalent in FMTs. HA and PCOS are related to early development of SM, IR, and other CVR factors with unknown consequences in adulthood.
La prevalencia de hiperandrogenismo (HA), que incluye el síndrome de ovario poliquístico (SOP), es alta en los pacientes transexuales de mujer a hombre (TMH). Este hecho se ha relacionado con el síndrome metabólico (SM), lo que parece aumentar la morbimortalidad cardiovascular a lo largo del tratamiento hormonal cruzado (THC).
ObjetivosDeterminar la prevalencia de HA y SOP en pacientes TMH antes del inicio del THC, y su asociación con el SM y sus componentes, la insulinorresistencia (IR) y otros factores de riesgo cardiovascular (RCV).
Materiales y métodosSetenta y siete TMH fueron valorados clínica y analíticamente para HA antes de iniciar el THC. También se determinaron los factores de RCV, la IR y otros parámetros del SM.
ResultadosLa prevalencia de HA fue del 49,4% (el 73,7% de ellos con SOP [criterios de Rotterdam]), y del total de la muestra el 36,4% presentaron SOP. La prevalencia global de SM fue del 38,4 y 51,7% (criterios ATP-III e IDF, respectivamente). Los pacientes con HA frente a aquellos sin HA presentaban SM (criterios ATP-III e IDF, respectivamente) en el 36,8 y 57,9% frente al 25,6 y 41% (p<0,0001 y p<0,01, respectivamente). El 54,5% de los pacientes tenía normopeso (índice de masa corporal [IMC] 18,5-24,9kg/m2), el 26% sobrepeso (IMC 25-29,9kg/m2) y el 19,5% eran obesos (IMC≥30kg/m2). Al ajustar por el IMC la comparación de parámetros hormonales, metabólicos y antropométricos mostró diferencias estadísticamente significativas en los valores de glucemia, HOMA-IR y perímetro abdominal (p<0,001), así como en los de colesterol-HDL (HDL) (p=0,033), pero no en las concentraciones de testosterona total o de testosterona libre calculada. Del total de la muestra el 27,3% presentaron niveles de HDL por debajo de 50mg/dl.
ConclusionesEl HA y el SOP son muy prevalentes en la población TMH. HA y SOP se relacionan con el desarrollo temprano de SM, IR y otros factores de RCV, de consecuencias desconocidas en la edad adulta.
Gender identity disorders (GIDs) occur when no correspondence exists between biological sex differentiation and gender self-perception. They are characterized by strong, persistent identification with the opposite sex and constant discomfort with the biological sex or the sexual behavior associated to the own sex. The etiology of GIDs is not clear, but endocrine, neuroanatomical, and psychosocial factors appear to be involved in all cases.1
Various studies have found a high prevalence of polycystic ovary syndrome (PCOS) in female-to-male (FTM) transsexual patients.1–5 It has been suggested that hyperandrogenism (HA) could be related to development of GIDs in FTM transexuals.5,6
PCOS is also the most prevalent cause of HA in FTM transsexuals. PCOS affects 5–10% of childbearing women.7,8 The most common clinical characteristics of this syndrome include: menstrual irregularity (oligo/anovulation) and signs of excess androgens such as hirsutism, alopecia, and acne.
PCOS is associated to significant metabolic changes. Several studies9,10 have reported a 10-fold greater prevalence of diabetes in women with PCOS, and that 30–50% of obese women with altered blood glucose levels (altered fasting blood glucose and glucose intolerance) have PCOS. PCOS is also related to an altered lipid profile and hypertension,11,12 which appears to increase cardiovascular risk in this population.
On the other hand, Gooren et al.13 found a high prevalence of components of the metabolic syndrome (MS) at the start of cross-sex hormone (CSH) treatment in FTM transsexuals, which appears to be related to the slight worsening in cardiovascular risk during treatment.
A study conducted at our unit4 showed changes at three months and one year of CSH therapy in anthropometric variables (weight increase) and cardiovascular risk factors (increased C reactive protein [CRP] and LDL cholesterol [LDL] levels and decreased HDL cholesterol [HDL] and apoprotein A [ApoA] levels).
The objective of this study was to assess the prevalence of HA and PCOS in FTM patients before the start of CSH therapy and their association to MS and its components, insulin resistance, and other cardiovascular risk factors (CVRFs).
Patients and methodsPatientsThis was a retrospective, descriptive study of 77 FTM patients aged 30±4.1 years (range: 18–43 yeas) diagnosed with gender dysphoria after psychological and endocrine assessment at the gender identity unit (GIU) of Hospital Ramón y Cajal from May 2007 to December 2008.
The study was approved by the ethics committee of the hospital, and all participants signed an informed consent. Clinical history data were collected. A gynecological ultrasound examination was performed in all patients. Standard laboratory tests at our unit, including the variables of interest for the study, were performed.
Diagnostic criteriaIn agreement with other authors2 who studied identical populations, biochemical HA or hyperandrogenemia was defined as calculated free testosterone (cFT) levels ≥0.028nmol/L, as established with the Vermeulen formula,14 Diagnosis of PCOS was based on the 2003 Rotterdam criteria, revised in 2004.15,16 At least two of the following criteria should be met: (1) oligo-ovulation or anovulation; (2) clinical and/or biochemical signs of HA; and (3) polycystic ovaries. Other causes of HA (non-classical congenital adrenal hyperplasia, thyroid dysfunction, hyperprolactinemia, Cushing syndrome, androgen-secreting tumors, syndrome of hyperandrogenism, insulin resistance, and acanthosis nigricans [HAIRAN], hyperthecosis, and acromegaly) should be ruled out first. The modified Ferriman–Gallwey scale was used to assess hirsutism.16 A value greater than 8 represents hirsutism (clinical HA). Oligo-ovulation is defined as irregular and scarce menses, and anovulation as no menstruation in the past 12 months. Ultrasound examination is considered to reveal polycystic ovaries when at least one follicle 10cc or greater in volume or 12 or more follicles 2–9mm in diameter are found. Diagnosis of MS was based on the ATP-III (2002) criteria17: abdominal circumference >88cm, triglycerides (TG)≥150mg/dL, HDL<50mg/dL, blood pressure (BP)≥130/85mmHg, and fasting blood glucose ≥110mg/dL; three or more of these are required to diagnose MS. The 2005 criteria of the International Diabetes Federation (IDF)18 were also used. These include an abdominal circumference ≥80cm plus two of the following: TG≥150mg/dL, HDL<50mg/dL, BP≥130/85mmHg, fasting blood glucose ≥100mg/dL.
Biochemical measurementsAll biochemical tests, except for hormone tests, were performed in a BM/Hitachi 717 autoanalyzer using enzymatic endpoint methods. Serum glucose, total cholesterol (TC), and TG levels were measured using an enzymatic colorimetric method. HDL particles were separated from TC by polyethylene glycol precipitation. LDL was calculated using the Friedewald formula. Patients with blood glucose levels ≥126mg/dL were considered to have diabetes, and patients with TC or TG levels >200mg/dL, HDL levels <35mg/dL and/or LDL levels >160mg/dL were considered as dyslipidemic.
IR was assessed using the homeostatic model of Matthews et al.,19 known as HOMA index (insulin [μU/mL]×glucose [mmol/L]/22.5). A HOMA-IR index ≥3.8 was considered to indicate IR, as proposed by Ascaso et al.20 Hormone measurements (follicle-stimulating hormone [FSH], luteinizing hormone [LH], estradiol, Δ4-androstenedione (Δ4-A): dehydroepiandrosterone sulfate [DHEA-S], prolactin, sex hormone binding globulin (SHBG), total testosterone (TT), insulin, and 17OH progesterone [17OHP]) were performed) using chemiluminescence methods (IMMULITE, DPC, LA, AC), except for TSH, which was measured by IRMA (Auto Delfia, Wallac, Gaithersburg, MD). Inter and intra-assay coefficients of variation for prolactin were 6.6% and 10.5% respectively. Pituitary magnetic resonance imaging was performed in patients with prolactin levels >100ng/mL; 17OHP stimulation with adrenocorticotropic hormone (ACTH) was done in cases with 17OHP levels >6nmol/L; and patients with TT levels >230ng/dL underwent CT of the adrenal glands.
Statistical analysisGoodness of fit of empirical to normal distribution was verified using a one-sample Kolmogorov–Smirnov (K–S) test, which was positive (with z=2.017 and p<0.001). Parametric analyses could therefore be used. Results are given as mean values and standard deviations. Data for various characteristics corresponding to subjects with and without HA were compared using a Student's t-test for independent samples. For MS components, percentages in the two groups with and without HA were compared using a Chi-square test through a contingency table. For metabolic and hormone parameters, the three groups created based on BMI were compared using one-factor ANOVA. Finally, the relationship between cFT level and four obesity markers was calculated using Pearson correlation coefficients. A value of p<0.05 was considered statistically significant. All calculations were made using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA).
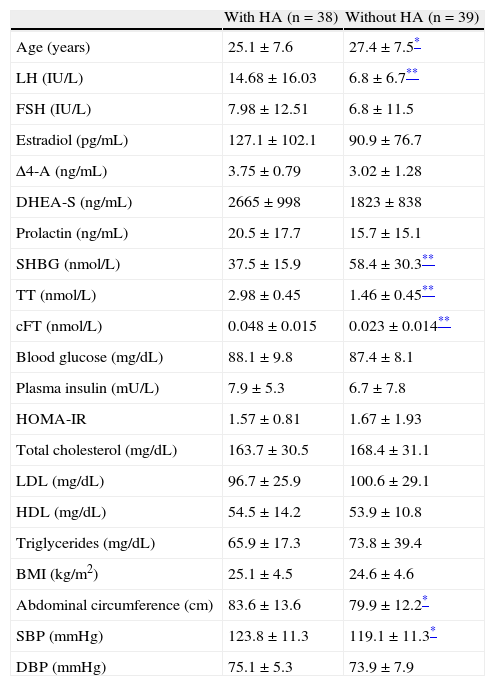
ResultsBased on cFT levels,2 38 of the 77 FTM patients (49.4%) participating in the study had HA. As regards the cause of HA, no patient has HAIRAN syndrome, congenital adrenal hyperplasia, or androgen-secreting tumors. Table 1 shows the hormonal, metabolic, and anthropometric characteristics differentiating patients with and without HA. Significant differences were found between both groups in age (p<0.05), LH, SHBG, TT and cFT levels (p<0.01 for all), and abdominal circumference and SBP (p<0.05).
Hormonal, metabolic, and anthropometric characteristics of subjects with and without HA.
| With HA (n=38) | Without HA (n=39) | |
| Age (years) | 25.1±7.6 | 27.4±7.5* |
| LH (IU/L) | 14.68±16.03 | 6.8±6.7** |
| FSH (IU/L) | 7.98±12.51 | 6.8±11.5 |
| Estradiol (pg/mL) | 127.1±102.1 | 90.9±76.7 |
| Δ4-A (ng/mL) | 3.75±0.79 | 3.02±1.28 |
| DHEA-S (ng/mL) | 2665±998 | 1823±838 |
| Prolactin (ng/mL) | 20.5±17.7 | 15.7±15.1 |
| SHBG (nmol/L) | 37.5±15.9 | 58.4±30.3** |
| TT (nmol/L) | 2.98±0.45 | 1.46±0.45** |
| cFT (nmol/L) | 0.048±0.015 | 0.023±0.014** |
| Blood glucose (mg/dL) | 88.1±9.8 | 87.4±8.1 |
| Plasma insulin (mU/L) | 7.9±5.3 | 6.7±7.8 |
| HOMA-IR | 1.57±0.81 | 1.67±1.93 |
| Total cholesterol (mg/dL) | 163.7±30.5 | 168.4±31.1 |
| LDL (mg/dL) | 96.7±25.9 | 100.6±29.1 |
| HDL (mg/dL) | 54.5±14.2 | 53.9±10.8 |
| Triglycerides (mg/dL) | 65.9±17.3 | 73.8±39.4 |
| BMI (kg/m2) | 25.1±4.5 | 24.6±4.6 |
| Abdominal circumference (cm) | 83.6±13.6 | 79.9±12.2* |
| SBP (mmHg) | 123.8±11.3 | 119.1±11.3* |
| DBP (mmHg) | 75.1±5.3 | 73.9±7.9 |
Δ4-A: Δ4-androstenedione; cFT: calculated free testosterone; DHEA-S: dehydroepiandrosterone sulfate; FSH: follicle-stimulating hormone; HA: hyperandrogenism; HDL-C: high density lipoprotein cholesterol; HOMA-IR: homeostasis model assessment-insulin resistance; BMI: body mass index; LDL-C: low density lipoprotein cholesterol; LH: luteinizing hormone; DBP: diastolic blood pressure; SBP: systolic blood pressure; SHBG: sex hormone binding globulin; TT: total testosterone. Data are given as mean±SD.
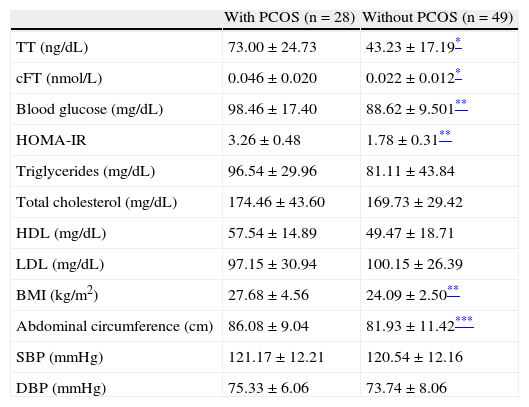
Using Rotterdam criteria, 28 of the 77 FTM patients in the study (36.4%) were diagnosed with PCOS. Of these patients, 18 (64.3%) had oligo/anovulation, 12 (42.8%) a gynecological ultrasound meeting PCOS criteria, and 6 (15.8%) hirsutism. Patients with PCOS had some significant differences from the group with no PCOS in TT and cFT levels (p<0.0001), blood glucose, HOMA-IR and BMI values (p<0.001 for all), and abdominal circumference (p<0.05) (Table 2).
Hormonal, metabolic, and anthropometric characteristics of subjects with and without PCOS.
| With PCOS (n=28) | Without PCOS (n=49) | |
| TT (ng/dL) | 73.00±24.73 | 43.23±17.19* |
| cFT (nmol/L) | 0.046±0.020 | 0.022±0.012* |
| Blood glucose (mg/dL) | 98.46±17.40 | 88.62±9.501** |
| HOMA-IR | 3.26±0.48 | 1.78±0.31** |
| Triglycerides (mg/dL) | 96.54±29.96 | 81.11±43.84 |
| Total cholesterol (mg/dL) | 174.46±43.60 | 169.73±29.42 |
| HDL (mg/dL) | 57.54±14.89 | 49.47±18.71 |
| LDL (mg/dL) | 97.15±30.94 | 100.15±26.39 |
| BMI (kg/m2) | 27.68±4.56 | 24.09±2.50** |
| Abdominal circumference (cm) | 86.08±9.04 | 81.93±11.42*** |
| SBP (mmHg) | 121.17±12.21 | 120.54±12.16 |
| DBP (mmHg) | 75.33±6.06 | 73.74±8.06 |
cFT: calculated free testosterone; HDL: high density lipoprotein cholesterol; HOMA-IR: homeostasis model assessment-insulin resistance; BMI: body mass index; LDL: low density lipoprotein cholesterol; DBP: diastolic blood pressure; SBP: systolic blood pressure; PCOS: polycystic ovary syndrome; TT: total testosterone.
Data are given as mean±SD.
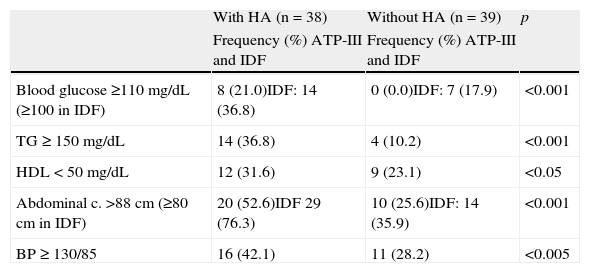
Based on ATP-III and IDF criteria, 29 and 40 of the 77 FTM patients (37.7% and 51.9% respectively) were diagnosed with MS. When adjusting for the presence or absence of HA, MS diagnosis was significantly different between both groups: 14 of the 38 patients with HA (36.8%) versus 10 of 39 (25.6%) (p<0.01) using ATP-III criteria, and 22 of the 38 patients with HA (57.9%) versus 16 of the 39 without HA (41%) (p<0.01) using IDF criteria. Table 3 shows the frequencies of MS components in both groups, with significantly different values for all of them.
Frequency of MS components in subjects with and without HA.
| With HA (n=38) | Without HA (n=39) | p | |
| Frequency (%) ATP-III and IDF | Frequency (%) ATP-III and IDF | ||
| Blood glucose ≥110mg/dL (≥100 in IDF) | 8 (21.0)IDF: 14 (36.8) | 0 (0.0)IDF: 7 (17.9) | <0.001 |
| TG≥150mg/dL | 14 (36.8) | 4 (10.2) | <0.001 |
| HDL<50mg/dL | 12 (31.6) | 9 (23.1) | <0.05 |
| Abdominal c. >88cm (≥80cm in IDF) | 20 (52.6)IDF 29 (76.3) | 10 (25.6)IDF: 14 (35.9) | <0.001 |
| BP≥130/85 | 16 (42.1) | 11 (28.2) | <0.005 |
ATP-III: Third Report of the National Cholesterol Education Expert Panel on Detection, Evaluation, and Treatment of high blood cholesterol in adults; HA: hyperandrogenism; HDL: high density lipoprotein cholesterol; IDF: International Diabetes Federation; BP: blood pressure; MS: metabolic syndrome; TG: triglycerides.
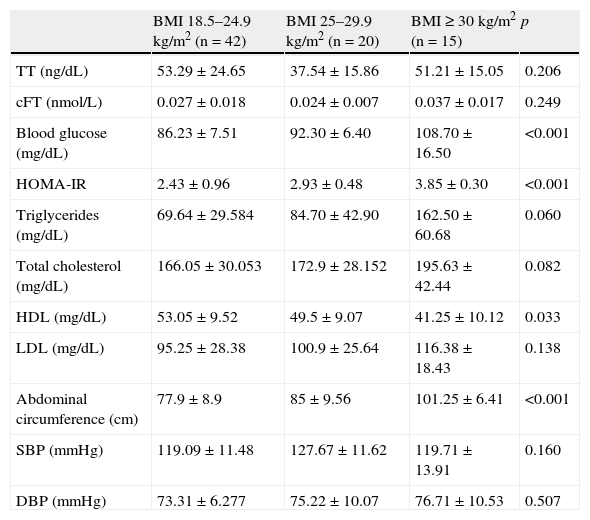
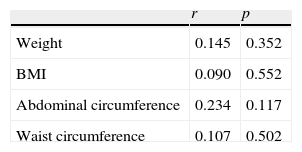
As regards body weight, 42 (54.5%) of the 77 patients had normal weight (BMI 18.5–24.9kg/m2), 20 (26%) were overweight (BMI 25–29.9kg/m2), and 15 (19.5%) were obese (BMI≥30kg/m2). When adjusting for BMI (Table 4), the comparison of some hormonal, metabolic, and anthropometric variables showed statistically significant differences in blood glucose, HOMA-IR, and abdominal circumference values (p<0.001 for all), and also in HDL levels (p=0.033). Differences were not significant for all other parameters. No correlation was also found for adiposity parameters and cFT (Table 5).
Hormonal, metabolic, and anthropometric characteristics adjusted for BMI.
| BMI 18.5–24.9kg/m2 (n=42) | BMI 25–29.9kg/m2 (n=20) | BMI≥30kg/m2 (n=15) | p | |
| TT (ng/dL) | 53.29±24.65 | 37.54±15.86 | 51.21±15.05 | 0.206 |
| cFT (nmol/L) | 0.027±0.018 | 0.024±0.007 | 0.037±0.017 | 0.249 |
| Blood glucose (mg/dL) | 86.23±7.51 | 92.30±6.40 | 108.70±16.50 | <0.001 |
| HOMA-IR | 2.43±0.96 | 2.93±0.48 | 3.85±0.30 | <0.001 |
| Triglycerides (mg/dL) | 69.64±29.584 | 84.70±42.90 | 162.50±60.68 | 0.060 |
| Total cholesterol (mg/dL) | 166.05±30.053 | 172.9±28.152 | 195.63±42.44 | 0.082 |
| HDL (mg/dL) | 53.05±9.52 | 49.5±9.07 | 41.25±10.12 | 0.033 |
| LDL (mg/dL) | 95.25±28.38 | 100.9±25.64 | 116.38±18.43 | 0.138 |
| Abdominal circumference (cm) | 77.9±8.9 | 85±9.56 | 101.25±6.41 | <0.001 |
| SBP (mmHg) | 119.09±11.48 | 127.67±11.62 | 119.71±13.91 | 0.160 |
| DBP (mmHg) | 73.31±6.277 | 75.22±10.07 | 76.71±10.53 | 0.507 |
HDL: high density lipoprotein cholesterol; HOMA-IR: homeostasis model assessment-insulin resistance; BMI: body mass index; LDL: low density lipoprotein cholesterol; DBP: diastolic blood pressure; SBP: systolic blood pressure.
PCOS is one of the most common endocrine diseases in women of childbearing age. It is essentially a hyperandrogenic disease.15 Its causes are not exactly known, but most experts agree in that it is a condition of a multifactorial origin where genetic and environmental factors which are only partially known are involved. An extensive investigation is therefore required to establish which causative elements are contributing to the occurrence of this clinical condition. In a recent study, Vassiliadi et al.21 found increased production and excretion of androgens and cortisol, as well as increased activity of 5α-reductase, which was not only explained by obesity, and postulated production of adrenal corticosteroids as a significant metabolic pathway in PCOS. In addition, up to 20% of women with PCOS show evidence of adrenal HA, with elevated DHEA-S levels.22 We also found in a previous study that DHEA-S levels were slightly higher in FTM transsexuals as compared to usual levels in women.4 A relative increase in cortisol and ACTH levels which could be related to hyperactivation of the adrenal axis in these patients was also seen. These findings could not be confirmed in our study patients, and no significant differences in DHEA-S levels depending on the presence or absence of HA were found either. It should also be noted that LH levels were significantly higher in the HA group. It should therefore be assumed that the source of high androgen levels in our FTM patients was mainly the ovary.
Limitations of our study include the lack of a control group, which is difficult to achieve except with a prospective design, which would be more complicated because of the complexity of the population to be studied. The impact of alcohol and smoking on tested parameters could also not be determined because information on these variables was not available in all cases, and this assessment was therefore ruled out in the initial analysis. In addition, our study used a marker obtained by calculation (cFT) rather than directly to diagnose HA. The reason for this was that we wanted to use the same parameter as other authors because of its greater diagnostic sensitivity2 and to be able to compare our results to those reported by them. An additional limitation could be the significant difference found in age as related to presence or absence of HA. However, we do not think that this may have influenced the overall results.
Strengths of this study include the fact that it was conducted in transsexuals, a difficult to recruit population. Prevalence of HA and PCOS in a larger sample than those of two recent studies on transsexual populations is reported, and also relates them to MS and other CVRFs. The Baba et al. study1 analyzed 69 Japanese FTM patients and found PCOS in 58% of them. By contrast, the Mueller et al. study2 on 61 German FTM patients found PCOS in only 14.8%. Prevalence in our study was 36.4%. This discrepancy does not appear to be attributable to methodological, but to genetic differences, as has been known for some time and recently reviewed by Escobar-Morreale et al.23 Unlike Mueller et al.,2 we did not include a control group of non-transsexual subjects, but data available from a Caucasian population (similar to ours) in our environment suggest a prevalence of 6.5%,7 much lower than found in our study and with no clear justification. A large prospective study comparing the prevalence of PCOS between FTM subjects and women of the general population would be needed.
On the other hand, the greater prevalence of PCOS in the Baba et al. study1 was not associated to a higher HA rate. In fact, these authors reported a lower rate as compared to us (39.1 versus 49.4%), but assessed this condition more rigorously by using four hormone parameters (TT, free testosterone, Δ4-A, and DHEA-S), while only one variable (cFT) was used in our study. However, Mueller et al.,2 using the same single parameter (cFT) as in our study, reported higher TT and cFT levels in the transsexual population as compared to the control group, with a HA rate of 44.3% which is more than double the rate in the control group of non-transsexual women and similar to our data. They stated that this high HA prevalence could possibly be explained by self-medication. However, this possibility was carefully investigated and ruled out in our study.
In addition to genetic factors, environmental factors such as obesity or intrauterine growth delay are involved in the etiopathogenesis of PCOS. No data are available on intrauterine growth delay, but data on obesity have been reported. According to some studies, obesity exerts its effect through the leptin–neuropeptide Y-axis, which may be associated to an increased opioid tone to modulate both gonadotropin secretion and insulin secretion by the pancreas.24 No significant correlation was found in our study (Table 5), but a slight trend was seen to increases in BMI and central distribution of adipose tissue.
One of the pathogenetic factors in PCOS reported in recent years is IR, and multiple genes involved in it have been identified.25 Young women with PCOS often have multiple CVRFs such as MS, type 2 diabetes mellitus, dyslipidemia, abdominal obesity, and arterial hypertension, and require prevention of future cardiovascular events. Increased adiposity is therefore common and promotes early atherosclerosis and cardiovascular mortality, representing a worldwide concern.10 FTM patients receive lifetime androgen treatment, which has uncertain long-term adverse consequences. If these patients also have PCOS, this treatment could significantly increase cardiovascular risk. Thus, in our study, patients with PCOS had significantly higher basal blood glucose, HOMA-IR, BMI, and abdominal circumference values than those without PCOS, in agreement with other studies.2
While a little over a half (54.5%) of our overall population had normal weight, only 7.2% of the PCOS subgroup had normal weight. By contrast, a majority (76.8%) of the population in the Baba et al. study1 had normal weight and, in contrast to our findings, most patients with PCOS (72.5%) had normal weight, a discrepancy which is difficult to explain. We cannot explain either why, despite the greater IR prevalence in their study as compared to ours (30.6% and 18.2% respectively), they found no difference in HOMA-IR between the groups with and without PCOS, whereas in our study HOMA-IR was not only different depending on the degree of obesity, but also on the presence or absence of PCOS, despite the fact that the cut-off point for HOMA-IR was almost twice higher in our study (3.8 versus 2). We also found differences in abdominal circumference and SBP related to the presence of PCOS or HA respectively. Such differences were not reported in the Baba et al. study,1 which may be explained by the lower number of obese patients or by the lower number of HOMA-IR measurements, which were not done in all patients (only in 49 of the 69 patients).
In addition, most FTM patients with PCOS in our study had overweight or obesity, mechanisms involved in the HA in these patients, and also in all other women of childbearing age. Cupisti et al.26 reached the same conclusion in their study, although other pathophysiological pathways of IR undoubtedly exist, including cytokines, tissue necrosis factor-alpha, leptin, resistin, etc. Thus, we cannot explain why not all patients with PCOS have HA, or why not all patients with HA are obese or have IR.
The relationship between impaired carbohydrate metabolism and excess androgens is well known. Some studies9,10 noted a high rate of glucose intolerance in women with hirsutism and normal androgen levels, and also in women with idiopathic HA. When MS prevalence was assessed in patients with and without HA, patients with HA were found to have a greater prevalence of impaired fasting blood glucose as compared to those with normal cFT levels (21% versus 0% respectively by ATP-III criteria, p<0.001). All five MS criteria were found in 26.3% of patients with HA. In the group with no HA, the criteria most commonly seen were BP≥130/85 and increased abdominal circumference. Overall, a greater prevalence of MS was seen in the HA group (36.8% versus 25.6%, p<0.001) with both MS criteria used (ATP-III and IDF).
Statistically significant differences were also found when adjusting hormonal, metabolic, and anthropometric parameters for BMI. We noted that the higher the BMI, regardless of cFT levels, the greater IR. In a study assessing adrenal function and metabolic changes in patients with idiopathic HA, Atmaca et al.27 reported that 16.1% of patients had impaired fasting blood glucose and/or glucose intolerance. Prevalence of IR in our series was 18.2%.
On the other hand, HDL level is a strong predictor of cardiovascular disease.28 HDL levels less than 50mg/dL were found in 27.3% of FTM patients. And although no differences were found in mean HDL levels between the groups with and without HA, low levels were significantly more common in the HA and, after adjustment for BMI, HDL levels were lower in the groups with overweight and obesity. Abdominal circumference was <80cm in most patients, but those with values >80cm had a poorer risk profile (greater IR, low HDL levels, and impaired blood glucose) for cardiovascular disease in the future. These findings are consistent with other studies conducted at our unit4 where FTM patients had a great number of inflammatory markers before CSH therapy and, especially, showed increased TC, LDL, apoprotein B and TG levels, as well as decreased HDL and ApoA levels, one year after starting CSH therapy.
In conclusion, our study suggests that HA and PCOS are highly prevalent in the FTM population. This may show involvement of a hormonal factor in the complex genesis of female to male transsexualism. HA and PCOS are related to development of MS and other CVRFs. As this is a young population, it should be taken into account that lifetime androgen treatment could worsen IR and other risk factors.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Becerra-Fernández A, Pérez-López G, Menacho Román M, Martín-Lazaro JF, Lucio Pérez MJ, Asenjo Araque N, et al. Prevalencia de hiperandrogenismo y síndrome de ovario poliquístico en transexuales de mujer a hombre. Endocrinol Nutr. 2014;61:351–358.