El Bierzo area is characterized by low urinary iodine levels in the pregnant population. Guidelines recommend that local reference values are established for the diagnosis of thyroid dysfunction in pregnancy. Our objectives were to establish reference values for thyroid-stimulating hormone (TSH), free thyroxine (FT4) and free triiodothyronine (FT3) in women in the first trimester of pregnancy and to explore the factors influencing variability in these hormones.
Patients and methodsA retrospective study of 412 women in the first trimester of pregnancy who were measured serum levels of TSH, FT4, and FT3; 163 women with conditions with a potential influence on thyroid function were excluded. Thirty smoking pregnant women were also excluded from the study of reference values. Factors examined in the variability study included age, body mass index (BMI), and smoking. A multifactorial analysis of covariance was performed.
ResultsReference values in first-trimester pregnant women were: TSH: 0.497–3.595mIU/L; FT4: 0.90–1.42ng/dL; FT3: 2.49–3.56pg/mL. TSH levels depended on mother age and on interaction between age and smoking. FT3 levels depended on the mother's BMI and smoking, and there was also an interaction between both factors.
ConclusionThe reference values found may be used to assess thyroid dysfunction in pregnant women from El Bierzo. TSH and FT3 levels are influenced by age and BMI of the mother and by smoking, in addition to the interaction of these factors.
La zona de El Bierzo se caracteriza por una baja yoduria en la población gestante. Las guías recomiendan establecer valores de referencia locales para el diagnóstico de la disfunción tiroidea en el embarazo. Los objetivos fueron obtener valores de referencia de tirotropina (TSH), tiroxina libre (T4L) y triyodotironina libre (T3L) en gestantes de primer trimestre y estudiar los factores que intervienen en la variabilidad de estas hormonas.
Pacientes y métodosEstudio retrospectivo de 412 gestantes con determinaciones en suero de TSH, T4L y T3L en el primer trimestre; se excluyeron 163 por condiciones con posible influencia sobre la función tiroidea. Para el estudio de los valores de referencia se excluyeron además 30 mujeres fumadoras. El estudio de la variabilidad se realizó para los factores edad, índice de masa corporal (IMC) y fumar, mediante análisis de la covarianza multifactorial.
ResultadosLos valores de referencia en gestantes de primer trimestre fueron: TSH: 0,497–3,595mUI/l; T4L: 0,90–1,42ng/dl; T3L: 2,49–3,56pg/ml. La TSH depende de la edad de la madre y de la interacción entre la edad y fumar. La T3L depende del IMC de la madre y de fumar, existiendo además una interacción entre ambos factores.
ConclusiónLos valores de referencia obtenidos pueden utilizarse para valorar la disfunción tiroidea en mujeres gestantes de El Bierzo. Los valores de TSH y T3L están influidos por la edad de la madre, el IMC y fumar, además de por las interacciones entre estos factores.
Pregnancy represents an exceptional challenge for the thyroid gland, and normal pregnancy involves complex changes in thyroid physiology, causing an increased risk of thyroid dysfunction.1 At least 5% of pregnant women experience some thyroid dysfunction.2 These dysfunctions are associated with complications of pregnancy, and also affect newborns. In pregnancy, they are mainly associated with abortion,3–5 premature delivery,4–6 postpartum anemia and bleeding,7 and increased fetal loss (odds ratio: 13.5).8 In newborns, complications include low weight and inadequate neurophysiological development, which may even cause a seven-point decrease in the intelligence quotient in children aged 7–9 years.9
Iodine is an essential element for the synthesis of thyroid hormones, and iodination levels in pregnant women are highly variable in Spain. The geographic area of El Bierzo is characterized by lower than recommended urinary iodine levels in women in the first trimester of pregnancy (≥150μg/L according to the World Health Organization),10 a median of 118μg/L, which causes a high prevalence of thyroid dysfunction in pregnant women.11,12 In one study only 15.6% of such women were found to be taking supplemental iodine.11
Reference thyroid hormone levels in pregnant women are different from those for the general population because of physiological changes in pregnancy, particularly in the first trimester. Since neurological fetal development occurs during that first trimester, any intervention should occur at the beginning of pregnancy to achieve a good neurological development. A diagnosis of thyroid dysfunction should therefore be made during the first trimester of pregnancy. For this, thyroid-stimulating hormone (TSH) should primarily be measured, and when this is altered, free thyroxine (FT4) and free triiodothyronine (FT3) should also be measured.
Several scientific societies, such as the American Thyroid Association (ATA) and the Endocrine Society, have recently published new guidelines for the diagnosis and management of thyroid dysfunction in pregnancy.13,14 A consensus document of the Spanish Society of Endocrinology and Nutrition (SEEN) intended to improve the diagnosis of a condition which is considered to be underdiagnosed is also available.15 There is some controversy among scientific bodies as to whether universal screening of thyroid hormones should be performed in the first trimester of pregnancy. The ATA and the Endocrine Society only recommend screening in high-risk patients,13,14 but in its document the SEEN advocates universal screening in Spain.15 The area of El Bierzo is defined as a high-risk area because of the low iodine intake, and all these societies would recommend the universal screening of pregnant women living in it. To diagnose thyroid dysfunction in pregnancy, all of these scientific bodies recommend verification that thyroid hormone levels are within the reference values for each trimester and area of the relevant laboratories.13–15 When such reference values are not available, they recommend what they consider to be adequate reference values. Specifically, SEEN and ATA recommend the use of 2.5μIU/mL as the cut-off value.
The purpose of this study was to establish reference values for TSH, FT4, and FT3 in women in the first trimester of pregnancy in our area that could be used for universal screening for thyroid dysfunction in the pregnant population. The study was also intended to find population factors which could explain the variability of TSH, FT4, and FT3 in pregnant women.
Subjects and methodsA retrospective study was conducted on 412 pregnant women consecutively attending Hospital del Bierzo from February 2011 to April 2012 for screening for chromosome diseases in the first trimester. The results of TSH, FT4, and FT3 measurements performed on serum samples between weeks 8 and 13 of pregnancy were collected. The clinical histories of the pregnant women and the database of chromosome disease screening were consulted to collect population data at the time of sampling for thyroid hormone measurement. The population data collected included age, weight, height, body mass index (BMI), prior and/or current diseases (thyroid and non thyroid), and the smoking of three or more cigarettes daily.
Pregnant women with endocrine changes, with no thyroid hormone data available between 8 and 13 weeks of pregnancy, with no information available on population data or prior diseases, and with twin and Down syndrome pregnancies were excluded from the study. Endocrine causes included treatment with thyroxine or other drugs altering thyroid hormones, positive anti-thyroid antibodies, a prior diagnosis of hypothyroidism or hyperthyroidism, or nutritional problems, and a diagnosis of diabetes mellitus or gestational diabetes. As smoking may alter thyroid hormone levels,16 pregnant women who smoked at least three cigarettes daily were excluded from the calculation of reference values in the first trimester.
TSH, FT4, and FT3 measurements were performed using an ADVIA CENTAUR XP analyzer from Siemens Healthcare Diagnostics®. TSH was measured using a double-antibody ultrasensitive assay with a range from 0.008 to 150mIU/L and with the following interassay coefficients of variation (iCV) for three levels: level 1=0.418mIU/L, iCV=2.1%; level 2=5.685mIU/L, iCV=2.4%; level 3=36.4mIU/L, iCV=3.1%. FT4 was measured using a competitive assay, with a range from 0.1 to 12ng/dL (1.3–155pmol/L) and iCV for three levels: level 1=0.883ng/dL, iCV=5.9%; level 2=2.155ng/dL, iCV=5.1%; level 3=4.1ng/dL, iCV=5.2%. FT3 was measured using a competitive assay, with a range from 0.2 to 20μg/mL (0.3–30.8μmol/L) and iCV for three levels: level 1=2.53μg/mL, iCV=3.2%; level 2=6.31μg/mL, iCV=2.2%; level 3=11.24μg/mL, iCV=3.4%.
Statistical analysisData were collected in Excel version 2003, and CBStat version 5.1 software was used to calculate reference values.17 Normal thyroid hormone distribution was analyzed using an Anderson–Darling test,18 and variables not normally distributed were transformed to adjust them to a parametric distribution.19 FT3 followed a parametric distribution, but FT4 required transformation by natural logarithms and TSH required dual exponential and modular transformation.20,21 Potential stratification by weeks of pregnancy, age, and BMI were studied,22 and possible outliers were analyzed to discard them or not.23 Reference values were established between the 2.5th and 97.5th percentiles, with 90% confidence intervals as recommended by the International Federation of Clinical Chemistry.24 Group comparison was performed using a Student's t test, a Mann–Whitney U test, a Kruskal–Wallis H test, or a one-factor ANOVA test as appropriate.
Thyroid hormone variability was analyzing using SPSS software (PASW Statistics 18®). Multivariate linear regression tests were done to obtain an approximation of significant variables, followed by a comprehensive study of multifactorial covariance (ANCOVA) for TSH, FT4, and FT3, in order to ascertain the main factors and interactions accounting for thyroid hormone variability. The variability factors analyzed included age ≥30 years, IMC≥30kg/m2, and smoking three or more cigarettes daily (smoker). TSH, FT4, or FT3 were introduced in the models as covariates if they were significant, in order to better differentiate the variability of the factors. FT4 was the covariate in the TSH model, while TSH was the covariate in the FT4 model and FT4 was the covariate in the FT3 model. Homogeneity and model lack-of-fit tests were performed, and residuals charts were analyzed to see whether the covariance models were correct. A value of p<0.05 was considered significant for all tests.
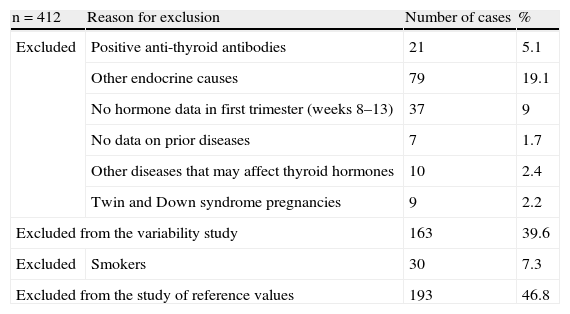
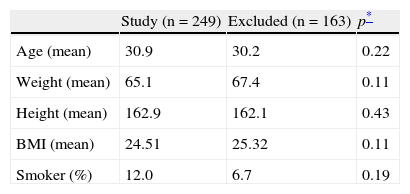
ResultsA total of 412 women aged 16–43 years in the first trimester of pregnancy were studied. Of these, 163 were excluded from the study of reference values, and 193 from the variability study. The reasons for exclusion are given in Table 1. The prevalence of anti-thyroid antibodies in pregnant women was 5.1%. Table 2 shows the characteristics of the study population, which were not significantly different between the pregnant women included and excluded.
Description of reasons for study exclusion.
| n=412 | Reason for exclusion | Number of cases | % |
| Excluded | Positive anti-thyroid antibodies | 21 | 5.1 |
| Other endocrine causes | 79 | 19.1 | |
| No hormone data in first trimester (weeks 8–13) | 37 | 9 | |
| No data on prior diseases | 7 | 1.7 | |
| Other diseases that may affect thyroid hormones | 10 | 2.4 | |
| Twin and Down syndrome pregnancies | 9 | 2.2 | |
| Excluded from the variability study | 163 | 39.6 | |
| Excluded | Smokers | 30 | 7.3 |
| Excluded from the study of reference values | 193 | 46.8 | |
Description of study population.
| Study (n=249) | Excluded (n=163) | p* | |
| Age (mean) | 30.9 | 30.2 | 0.22 |
| Weight (mean) | 65.1 | 67.4 | 0.11 |
| Height (mean) | 162.9 | 162.1 | 0.43 |
| BMI (mean) | 24.51 | 25.32 | 0.11 |
| Smoker (%) | 12.0 | 6.7 | 0.19 |
BMI, body mass index.
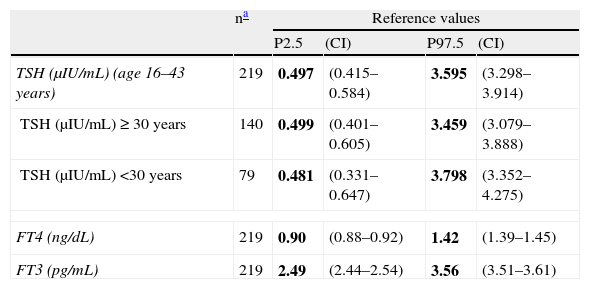
Table 3 shows the reference values obtained in the first trimester of pregnancy for TSH, FT4, and FT3, with their corresponding confidence intervals. Upon stratification between weeks 8 and 13 of pregnancy, no significant differences were found between pregnancy weeks in TSH (p=0.502), FT4 (p=0.979), and FT3 (p=0.564), applying one-factor ANOVA taking into account the means of these parameters in each week (data not shown). A significant age-related decrease in TSH was seen (p<0.001). Table 3 also shows TSH reference values for pregnant women aged <30 years and ≥30 years. A significant BMI-related increase in FT3 was also seen (p<0.001), but no stratification was made because of the small number of cases with BMI≥30kg/m2 (n=24).
Reference values in women in the first trimester of pregnancy (weeks 8–13) in El Bierzo.
| na | Reference values | ||||
| P2.5 | (CI) | P97.5 | (CI) | ||
| TSH (μIU/mL) (age 16–43 years) | 219 | 0.497 | (0.415–0.584) | 3.595 | (3.298–3.914) |
| TSH (μIU/mL)≥30 years | 140 | 0.499 | (0.401–0.605) | 3.459 | (3.079–3.888) |
| TSH (μIU/mL) <30 years | 79 | 0.481 | (0.331–0.647) | 3.798 | (3.352–4.275) |
| FT4 (ng/dL) | 219 | 0.90 | (0.88–0.92) | 1.42 | (1.39–1.45) |
| FT3 (pg/mL) | 219 | 2.49 | (2.44–2.54) | 3.56 | (3.51–3.61) |
CI, confidence interval; P, percentile; TSH, thyroid-stimulating hormone; FT3, triiodothyronine not bound to protein; FT4, thyroxine not bound to protein.
Median urinary iodine levels in women in the first trimester of pregnancy in El Bierzo: 118μg/L.
Reference values appear in bold.
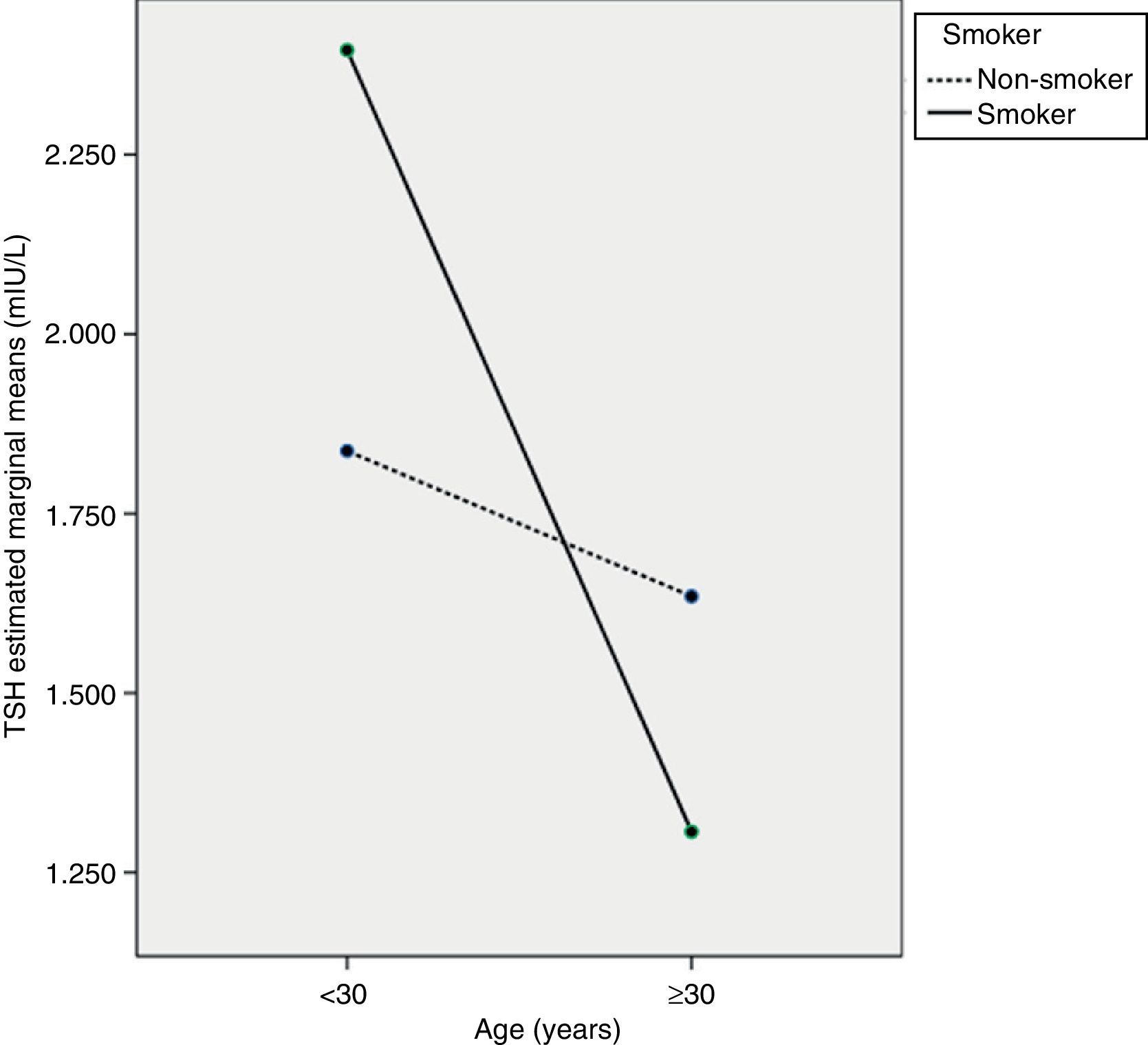
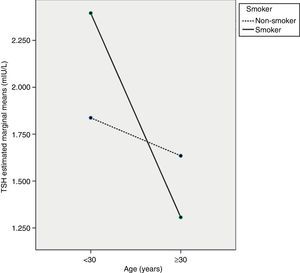
The ANCOVA test showed that TSH decreased with age, mainly in pregnant women ≥30 years (p<0.05), but that TSH levels in pregnant smokers did not differ from those in non-smokers (p=0.471). However, an interaction was seen to exist between the two variables, so that women ≥30 years of age who smoked had the lowest TSH levels (p<0.05). Fig. 1 shows a profile plot of the marginal means estimated by the TSH model.
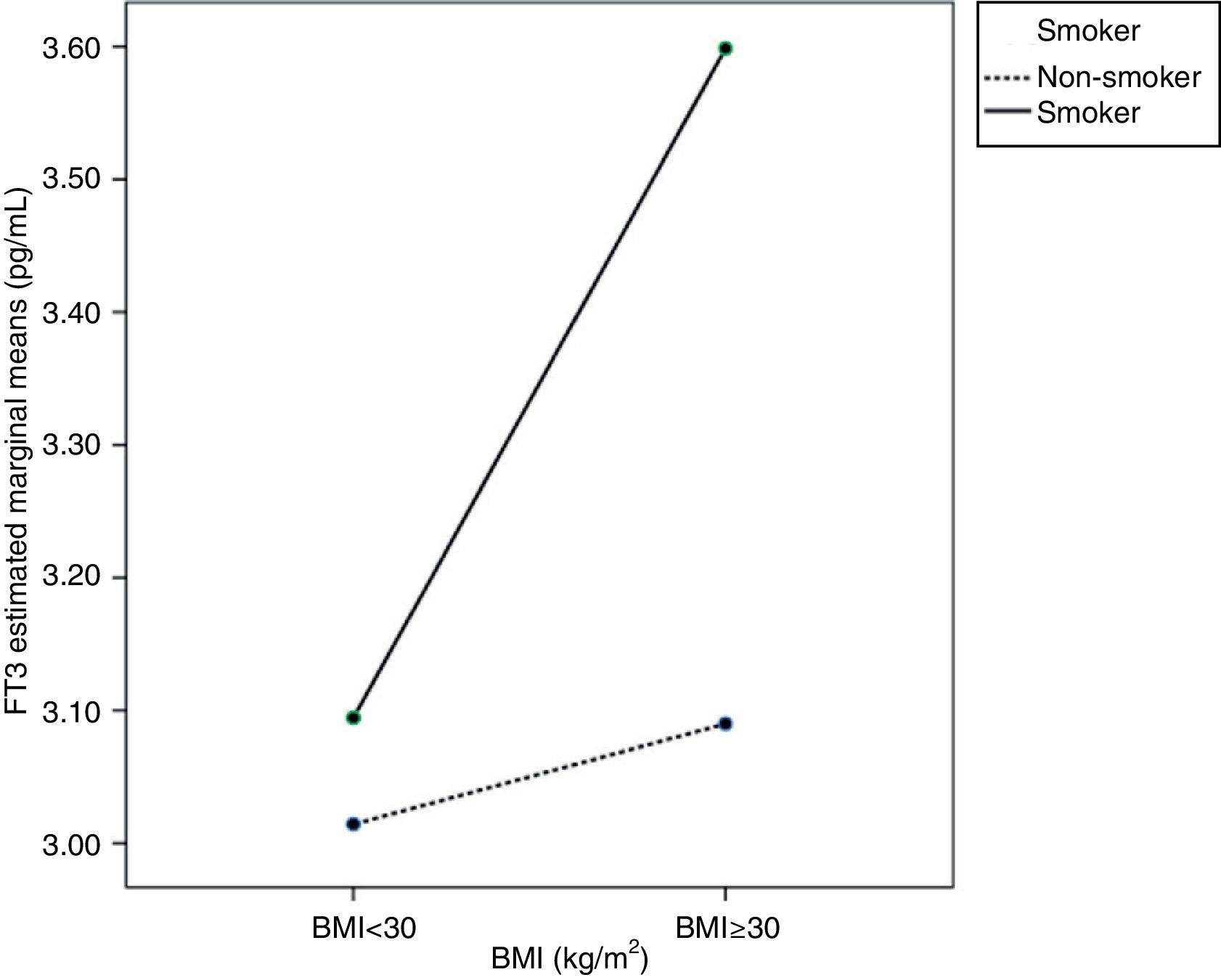
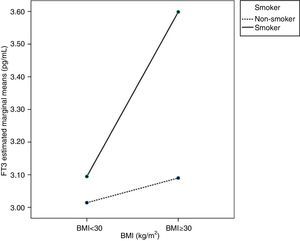
Age, BMI, or smoking had no influence on FT4 variability. As regards FT3, ANCOVA showed that FT3 increased with BMI, mainly in pregnant women with BMI≥30kg/m2 (p<0.05). Smoking also increased FT3 levels in pregnant women (p<0.05). An interaction was also seen between BMI≥30kg/m2 and smoking, so that pregnant women who smoked and had BMIs≥30kg/m2 (p<0.05) had higher FT3 levels. This interaction is better seen in the profile plot (Fig. 2).
DiscussionThe issue of the various recommendations made by scientific bodies and the lack of reference values for TSH, FT4, and FT3 in El Bierzo, an area where a high proportion of pregnant women have iodine deficiency, led us to calculate more precisely the reference values for those hormones in the first trimester of pregnancy. Although this was a retrospective study, maximum precautions were taken in our exclusion criteria. Any pregnant woman in whom there was the least doubt as to whether a treatment or disease would influence thyroid function was excluded from the study. This explains the high proportion of women excluded (39.6%). However, as shown in Table 2, pregnant women included in and excluded from the study did not differ in the population data, and a homogeneous population could be assumed.
To calculate reference values, distributions were normalized to obtain reference values with confidence intervals as adjusted as possible, which made the number of individuals less relevant. The upper TSH reference value (97.5th percentile=3.595μIU/mL) was somewhat higher than 2.5μIU/mL, the value recommended by the guidelines if no local reference values are available.13–15 Some authors relate this higher value to a low iodine intake in the population,25 but others think that no association exists between urinary iodine and TSH levels in the first trimester of pregnancy.26 Despite the stratification by age of pregnant women for subsequent use in screening for thyroid dysfunction in the first trimester of pregnancy, we think that it would be advisable to define the reference values of the total population, with no stratification by age, because it is more practical.
Since TSH and FT4 and FT3 levels did not differ between weeks 8 and 13 of pregnancy, a differentiation of reference values by week is not required, and they are grouped as reference values for the first trimester. Some reports provide reference values by weeks of pregnancy,27,28 while others do not,29,30 but even García de Guadiana Romualdo et al.,27 who give values according to gestational age, state that there are no significant differences in TSH by gestational week in the first trimester.
A direct comparison of reference values found in this and other studies, even with the same procedures, is very difficult for several reasons. Many retrospective population studies use positive anti-thyroid antibodies as the only criterion, but as suggested by Spencer et al.,31 this procedure biases the upper reference TSH value due to other occult thyroid dysfunctions. Other studies used populations that were not comparable to ours both with regard to race25 and because the nutritional iodine status of the population had not been studied.28 Moreover, there is no recognized reference method for the standardization of free thyroid hormone tests.
The ANCOVA test showed that TSH levels of pregnant women are influenced by age and by interaction between age and smoking. As in some studies, smoking alone did not result in different TSH levels,16 although differences were found in other studies.32,33 As regards age as an isolated factor, there are no reports in the literature suggesting that TSH levels decrease with an increase in the age of pregnant women, in particular from 30 years of age. Age-related increases31 or decreases34 in TSH levels have however been seen in the general population. Specifically, Völzke et al.34 hypothesized that an inverse relationship between TSH and age is seen in iodine-deficient populations, presumably due to prevailing nodular disease and increased thyroid autonomy with age. No references suggesting a potential interaction between age and smoking that causes pregnant women aged ≥30 years who are smokers to have lower TSH levels have been found either. This is a new finding which should be investigated in other studies so as to confirm it with a greater number of cases. If this finding is confirmed, TSH reference values in the first trimester of pregnancy for thyroid dysfunction screening will have to be stratified according to the age and smoking status of the women.
As regards FT4 levels, they were not influenced by age ≥30 years, BMI≥30kg/m2, or smoking. Other studies also reached the same conclusion with regard to smoking.16
As regards FT3 levels, other studies suggested that they increase with BMI35,36 and also with smoking,16 but independently. No study reported an interaction between BMI≥30kg/m2 and smoking, as seen in this study. This interaction may be due to increased peripheral metabolism induced by smoking16 and increased by the greater body surface area.
We conclude that the reference values obtained for TSH, FT4, and FT3 may be used for thyroid dysfunction screening in the first trimester of pregnancy in El Bierzo. TSH levels in the first trimester of pregnancy are influenced by the age of the mother and by the interaction between age and smoking. FT3 levels in the first trimester of pregnancy depend on BMI and the smoking status of the mother, and an interaction between both factors also exists.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Lombardo Grifol M, Gutiérrez Menéndez ML, García Menéndez L, Valdazo Revenga MV. Valores de referencia y estudio de la variabilidad de hormonas tiroideas en gestantes de El Bierzo. Endocrinol Nutr. 2013;60:549–554.