To assess whether sleep apnea-hypopnea syndrome (SAHS) is a risk factor for development of acromegalic cardiomyopathy.
MethodsA descriptive, cross-sectional study of 32 patients with acromegaly (15 categorized as non-controlled-NCA and 17 as controlled-CA) compared to 20 matched controls (by sex, age, and BMI) referred to the pulmonology department for suspected SAHS. Polysomnography, echocardiography (M-mode, 2-dimensional, and Doppler), and 12-lead electrocardiography were performed in all participants. Development of cardiac morbidity (ischemia heart disease or heart failure) was evaluated after 7 years.
ResultsSAHS was diagnosed in 81.3% of patients with acromegaly and 85% of controls. Mild SAHS was more common in CA than in NCA patients (31.3% vs. 0%, p=0.048). There was a trend to greater prevalence of left ventricular diastolic dysfunction (LVDD) in acromegalic patients as compared to controls (58.1% vs. 30%, p=0.05). Presence of severe SAHS in patients with acromegaly was related to greater risk of LVDD (90.9% vs. 40%, p=0.008; OR 2.3 [1.3–4.0]), LV hypertrophy (55.6% vs. 10.5%, p=0.02; OR 5.3 [1.3–22.2]), and cardiac events (87.5% vs. 35.6%; p=0.01; OR 7.53 [1.07–53.24]).
ConclusionSAHS is highly prevalent in patients with acromegaly. Only in these patients was severe SAHS associated to hypertrophy, LV diastolic dysfunction, and cardiac events.
La acromegalia se asocia con el síndrome de apnea-hipopnea del sueño (SAHS) y cambios a nivel cardíaco. Nuestro objetivo es evaluar si la presencia de SAHS es un factor de riesgo de desarrollo de cardiomiopatía acromegálica.
Material y métodoEstudio transversal descriptivo de 32 pacientes acromegálicos (15 clasificados como no-controlados–NCA- y 17 como controlados-CA-) comparados con 20 controles pareados (en sexo, edad e IMC) derivados al Servicio de Neumología por sospecha de SAHS. Se realizó polisomnografía, ecocardiografía (M-modo, 2-dimensiones, y Doppler) y electrocardiograma de 12-derivaciones a todos los participantes. Tras 7 años, se evaluó el desarrollo de morbilidad cardiológica (isquemia o insuficiencia cardíacas reportadas).
Resultados81,3% pacientes acromegálicos y 85% controles se diagnosticaron de SAHS. SAHS leve fue más frecuente en CA que NCA (31,3% vs. 0%, p=0,048). Existía una tendencia a mayor prevalencia de disfunción diastólica del ventrículo izquierdo (DDVI) en los pacientes acromegálicos comparados con los controles (58,1% vs. 30%, p=0,05). La presencia de SAHS grave en los pacientes acromegálicos se relacionó con mayor riesgo de DDVI (90,9% vs. 40%, p=0,008; OR 2,3 [1,3–4,0]), hipertrofia del VI (55,6% vs. 10,5%, p=0,02; OR 5,3 [1,3–22,2]) y eventos cardíacos (87,5% vs. 35,6%; p=0.01; OR 7.53 [1.07–53.24]).
ConclusionesSAHS es muy frecuente en los pacientes acromegálicos. Sólo en pacientes acromegálicos, el SAHS grave se asoció con hipertrofia, disfunción diastólica del VI y eventos cardíacos.
Sleep apnea–hypopnea syndrome (SAHS) is characterized by episodes of interruption of respiration associated with fragmented sleep and hypoxia. It can be divided into obstructive sleep apnea (OSA), defined by recurrent episodes of either partial or complete upper airway obstruction during sleep, and central sleep apnea (CSA), with reductions in central respiratory drive.1 SAHS is frequently associated with cardiovascular diseases in general population. It has been described that 40% of patients with hypertrophic cardiomyopathy also have SAHS and it is related to left atrial and aortic enlargement.2 It is also a frequent association between SAHS and heart failure. The negative intrathoracic pressure produced during obstructive apneas increases left ventricular (LV) after load, reduces cardiac output, and may promote the progression of heart failure. Moreover, intermittent hypoxia and reoxygenation cause vascular endothelial damage and could be associated with coronary artery disease and ischemic cardiomyopathy.3 Indeed, it has been proven that SAHS, in particular central sleep apnea, is an independent predictor of readmission in hospitalized patients with systolic heart failure.4 Furthermore several investigations reported an increasing frequency of cardiac arrhythmias among SAHS patients as consequence structural myocardial changes induced by recurrent hypoxemias and an increased sympathetic activity.2,5
Acromegaly is a rare disease, with an estimated prevalence around 69 cases per million inhabitants, characterized by an excess of growth hormone that causes important morbidity affecting cardiac and respiratory systems among others.1 Acromegaly induces myocardial hypertrophy and fibrosis of left ventricle, causing diastolic and more rarely systolic dysfunction and arrhythmias.6 It is known that cardiac involvement is the major determinant of the shortened life expectancy in these patients.7 There has been described a prevalence around 36–58% of left ventricle diastolic dysfunction (LVDD).7,8 On the other hand, SAHS affects 67–75% of acromegalic patients, although higher prevalence has been described.1 In acromegaly, OSA is more frequent than CSA due to craniofacial deformations, hypertrophy of pharyngeal soft tissue, macroglossia and thickening of upper airway. CSA is caused by central inhibition of breathing center by elevated levels of GH/IGF-1 serum levels.9
Some studies have explored the association between SAHS and cardiovascular outcomes in general population,2–5 but there are few data referred to acromegaly. Our purpose was to evaluate if SAHS is a risk factor to myocardiopathy on acromegalic patients.
Materials and methodsIt was designed a prospective observational descriptive study of 32 acromegalic patients (14 men, 50.3±11.4 years, 29.4±4.8kg/m2) treated in the Endocrinology Department of Hospital General Universitario de Alicante. The study was offered to all acromegalic patients without known SAHS that visited our department in 2007. The diagnosis of acromegaly was made based on elevated age-adjusted IGF-1 levels and lack of suppression of GH to less than 1ng/mL after oral glucose testing. Patients were classified as non-controlled acromegaly (15 patients, NCA) or controlled acromegaly (17 patients, CA) according to cure criteria published on 2000.10 Patients with known cardiopathy were excluded. On CA group, 52.3% patients were controlled due to somatostatin analogs. Disease duration was defined as the time interval between diagnosis was done and patient recruitment or disease control in CA group. It was recorded previous and current acromegaly treatments (surgery, radiotherapy, somatostatin analogs – SA and receptor antagonist). We selected 20 patients referred to the Respiratory Department for SAHS study as controls, in order to have a similar prevalence to acromegaly group. They were paired with acromegalic patients in sex, age and BMI (10 men, 53.2±12.7 years, 31.6±6.6kg/m2). There was no clinical suspicion of acromegaly in controls. We evaluated presence of comorbidities that could act as confounding factors, like hypertension, hypercholesterolemia, diabetes mellitus, thyroid dysfunction (goitier, hypo or hyperthyroidism), obesity, previous cardiovascular or respiratory disease and smoking status. We confirm cardiological morbidity (as ischemic cardiac event or cardiac insufficiency) during seven years of follow-up. This study was approved by the Local Ethics Committee and informed consent was obtained from each patient.
Polysomnography was performed using a Somnostar α system (SensorMedics Co., Yorba Linda, California, USA) that recorded the following: electroencephalogram (4 channels: C3-A2, O1-A2, C2-A1, and O21), electro-oculogram, submental and tibial electromyogram, electrocardiogram, thoracic, and abdominal movement (to determine respiratory effort) using piezoelectric belts, airflow using both an nasal cannula connected to a pressure transducer (Protech, Minneapolis, MN) and a thermistor airflow sensor with type E thermocouple technology, (sensitivity, 2–30μV/mm), changes in arterial oxygen saturation using a SensorMedics pulse oximeter (SensorMedics Co., Yorba Linda, CA, USA) (standard error of oxygen saturation in arterial blood of ±2%; of pulse, 1beat/min, with sampling every 6s), snoring using a piezo crystal sensor, and body position using a gold-plated ball bearing rotary sensor. The following criteria were used for PSG studies: apnea (cessation of oronasal airflow lasting 10s or more) and hypopnea (decrease in airflow of 50% or more for at least 10s, associated with arousals and/or a decline in arterial oxygen saturation of at least 3%). Sleep stages were classified according to the guidelines recommended by Rechtschaffen and Kales.11 The ASDA definition of arousals was used.12 Patients were defined having sleep-apnea (SAHS) if they had more than five apneas or hypopnoeas per hour. Severity of SAHS was graded as mild if there were recorded 5–15 events per hour, moderate between 15 and 30 events per hour or severe if there were more than 30 events per hour.
Echocardiography M-mode, 2-dimensional, and Doppler echocardiographic studies were performed (Hewlett-Packard Sonos 5500, Hewlett-Packard Corporation, Andover, MA) using a 2.5-MHz transducer during 3 consecutive cardiac cycles by the same researcher. All examinations were performed according to the recommendations of the American Society of Echocardiography. The LV ejection fraction was estimated according to Simpson's method. LV mass was calculated using Devereux's formula, 20 according to the Penn convention. LV hypertrophy was considered when LV mass index was 135g/m2 in men and 110g/m2 in women. Doppler studies provided indexes of ventricular filling that were derived from the mitral flow velocity curves: the E (early) and A (late) waves (m/s), the E/A ratio, E-wave deceleration time, and isovolumetric relaxation time. All measurements were taken using pulsed Doppler from the apical 4-chamber view. Diastolic dysfunction was defined as an E/A ratio 1.0, E-wave deceleration time 220ms, and isovolumetric relaxation time 100ms. Extrasystolic and postextrasystolic beats were rejected because of altered hemodynamics. At study entry, all subjects underwent 12-lead electrocardiography, being classified as normal or abnormal (combined of LV hypertrophy, branch bundle block or arrhythmia).
Statistical analysis: Variables were tested for normal distribution with the Kolmogorov–Smirnov test. We used Student's t/U-Mann–Whitney test for comparisons between two groups (total acromegaly and control group, NCA and CA) or ANOVA/Kruskall–Wallis for more than two groups. Categorical variables were compared with Chi-square or Fisher tests. For multivariate analysis we used multiple linear regression between continuous variables and unconditional logistic regression between categorical variables. A p<0.05 was considered significant. Statistical analysis was performed using SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
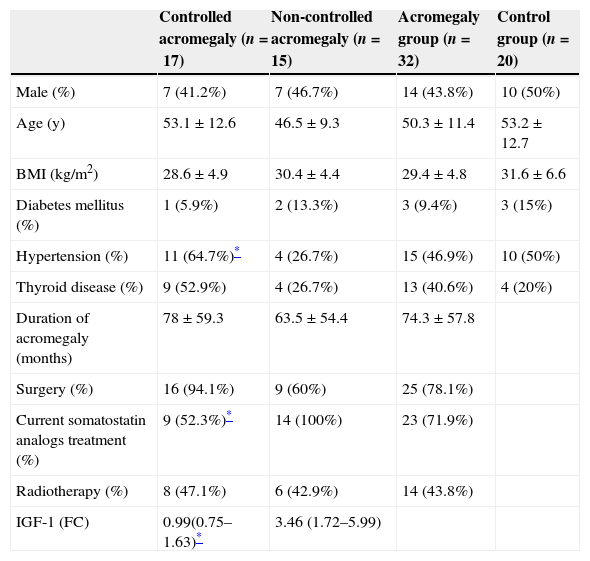
ResultsThere were no differences on sex, age, BMI, duration of acromegaly or presence of diabetes mellitus between CA and NCA groups. Current treatment with somatostatin analogs was more frequent on NCA (100% vs. 52.3%, p=0.003) as well as more CA patients had undergone to surgery (94.1% vs. 60%, p=0.03). No patient was treated with pegvisomant. Hypertension was more prevalent on CA (64.7 vs. 26.7%, p=0.03), although there were no differences between acromegalic patients and controls (46.9% vs. 50%, p=0.83). Table 1 resumes baseline characteristics.
Baseline characteristics.
| Controlled acromegaly (n=17) | Non-controlled acromegaly (n=15) | Acromegaly group (n=32) | Control group (n=20) | |
|---|---|---|---|---|
| Male (%) | 7 (41.2%) | 7 (46.7%) | 14 (43.8%) | 10 (50%) |
| Age (y) | 53.1±12.6 | 46.5±9.3 | 50.3±11.4 | 53.2±12.7 |
| BMI (kg/m2) | 28.6±4.9 | 30.4±4.4 | 29.4±4.8 | 31.6±6.6 |
| Diabetes mellitus (%) | 1 (5.9%) | 2 (13.3%) | 3 (9.4%) | 3 (15%) |
| Hypertension (%) | 11 (64.7%)* | 4 (26.7%) | 15 (46.9%) | 10 (50%) |
| Thyroid disease (%) | 9 (52.9%) | 4 (26.7%) | 13 (40.6%) | 4 (20%) |
| Duration of acromegaly (months) | 78±59.3 | 63.5±54.4 | 74.3±57.8 | |
| Surgery (%) | 16 (94.1%) | 9 (60%) | 25 (78.1%) | |
| Current somatostatin analogs treatment (%) | 9 (52.3%)* | 14 (100%) | 23 (71.9%) | |
| Radiotherapy (%) | 8 (47.1%) | 6 (42.9%) | 14 (43.8%) | |
| IGF-1 (FC) | 0.99(0.75–1.63)* | 3.46 (1.72–5.99) |
y, years; FC, fold change referred to standard deviation score for IGF-1. Data shown as median (percentile 25–75) and absolute number (percentage).
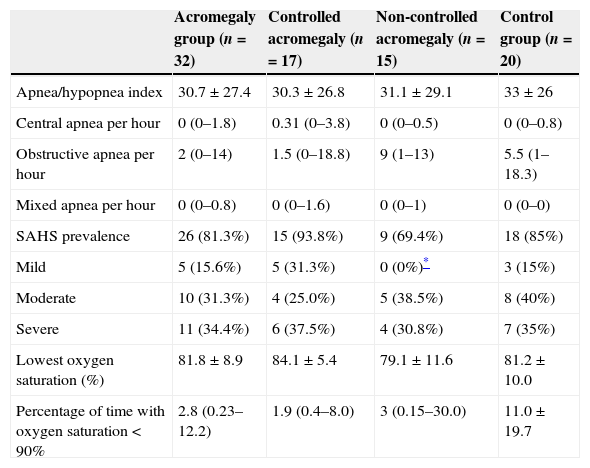
Table 2 shows major polysomnographic data. Twenty-six acromegalic patients (81.3%) had SAHS (15.6% mild, 31.4% moderate and 34.4% severe). On control group there were similar results according to SAHS prevalence, eighteen patients (85%) demonstrate SAHS (15% mild, 40% moderate and 35% severe).
Polysomnographic findings.
| Acromegaly group (n=32) | Controlled acromegaly (n=17) | Non-controlled acromegaly (n=15) | Control group (n=20) | |
|---|---|---|---|---|
| Apnea/hypopnea index | 30.7±27.4 | 30.3±26.8 | 31.1±29.1 | 33±26 |
| Central apnea per hour | 0 (0–1.8) | 0.31 (0–3.8) | 0 (0–0.5) | 0 (0–0.8) |
| Obstructive apnea per hour | 2 (0–14) | 1.5 (0–18.8) | 9 (1–13) | 5.5 (1–18.3) |
| Mixed apnea per hour | 0 (0–0.8) | 0 (0–1.6) | 0 (0–1) | 0 (0–0) |
| SAHS prevalence | 26 (81.3%) | 15 (93.8%) | 9 (69.4%) | 18 (85%) |
| Mild | 5 (15.6%) | 5 (31.3%) | 0 (0%)* | 3 (15%) |
| Moderate | 10 (31.3%) | 4 (25.0%) | 5 (38.5%) | 8 (40%) |
| Severe | 11 (34.4%) | 6 (37.5%) | 4 (30.8%) | 7 (35%) |
| Lowest oxygen saturation (%) | 81.8±8.9 | 84.1±5.4 | 79.1±11.6 | 81.2±10.0 |
| Percentage of time with oxygen saturation<90% | 2.8 (0.23–12.2) | 1.9 (0.4–8.0) | 3 (0.15–30.0) | 11.0±19.7 |
We did not find statistical differences on prevalence of SAHS between CA and NCA groups (93.8% vs. 69.2%, p=0.14), although mild SAHS was more frequent on CA than NCA patients (31.3% vs. 0%, p=0.048). The severity of SAHS was not related to the time of evolution of acromegaly, IGF-1 levels or SSA treatment. On multivariate analysis, CA was related to mild SAHS as independent variables.
Electrocardiographic changesAcromegalic group had a trend toward to more abnormalities on ECG (LV hypertrophy, branch bundle block or arrhythmia) than control group (39.3% vs. 15%, p=0.068). All patients with ECG changes had SAHS, both in acromegalic (p=0.1, OR 1.42 [1.04–1.93]) and control group (p=1.0, OR 1.21 [0.97–1.51]), although without statistical significance. There were no differences between activity status of acromegaly or SSA treatment.
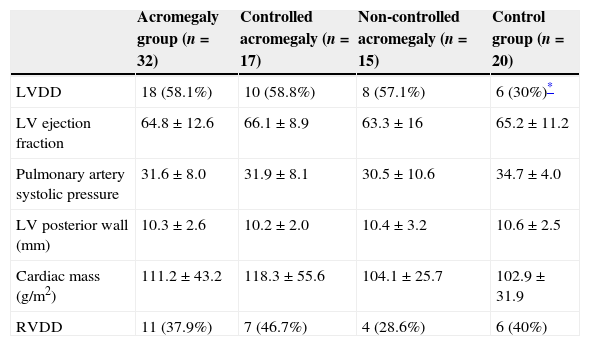
Echocardiographic changesTable 3 resumes major echocardiographic findings. We found a trend toward to more prevalence of left ventricle diastolic dysfunction (LVDD) in acromegalic patients compared to controls (58.1% vs. 30%, p=0.05). There were no differences on LVDD prevalence between CA and NCA groups (58.8% vs. 57.1%, p=1.0). We did not find echocardiographical differences depending on SSA treatment.
Echocardiographic findings.
| Acromegaly group (n=32) | Controlled acromegaly (n=17) | Non-controlled acromegaly (n=15) | Control group (n=20) | |
|---|---|---|---|---|
| LVDD | 18 (58.1%) | 10 (58.8%) | 8 (57.1%) | 6 (30%)* |
| LV ejection fraction | 64.8±12.6 | 66.1±8.9 | 63.3±16 | 65.2±11.2 |
| Pulmonary artery systolic pressure | 31.6±8.0 | 31.9±8.1 | 30.5±10.6 | 34.7±4.0 |
| LV posterior wall (mm) | 10.3±2.6 | 10.2±2.0 | 10.4±3.2 | 10.6±2.5 |
| Cardiac mass (g/m2) | 111.2±43.2 | 118.3±55.6 | 104.1±25.7 | 102.9±31.9 |
| RVDD | 11 (37.9%) | 7 (46.7%) | 4 (28.6%) | 6 (40%) |
LVDD: left ventricle diastolic dysfunction; LV: left ventricle; RVDD: right ventricle diastolic dysfunction.
Data shown as median (percentile 25–75) and absolute number (percentage).
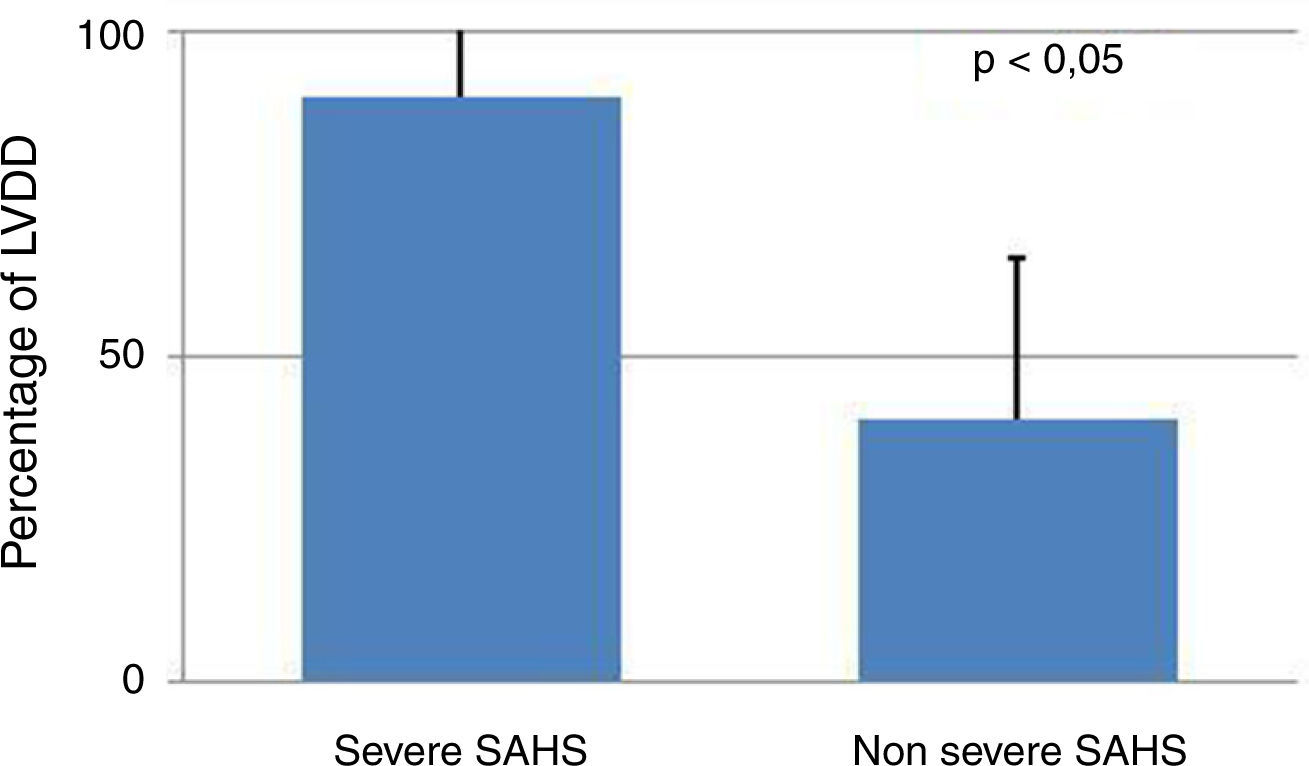
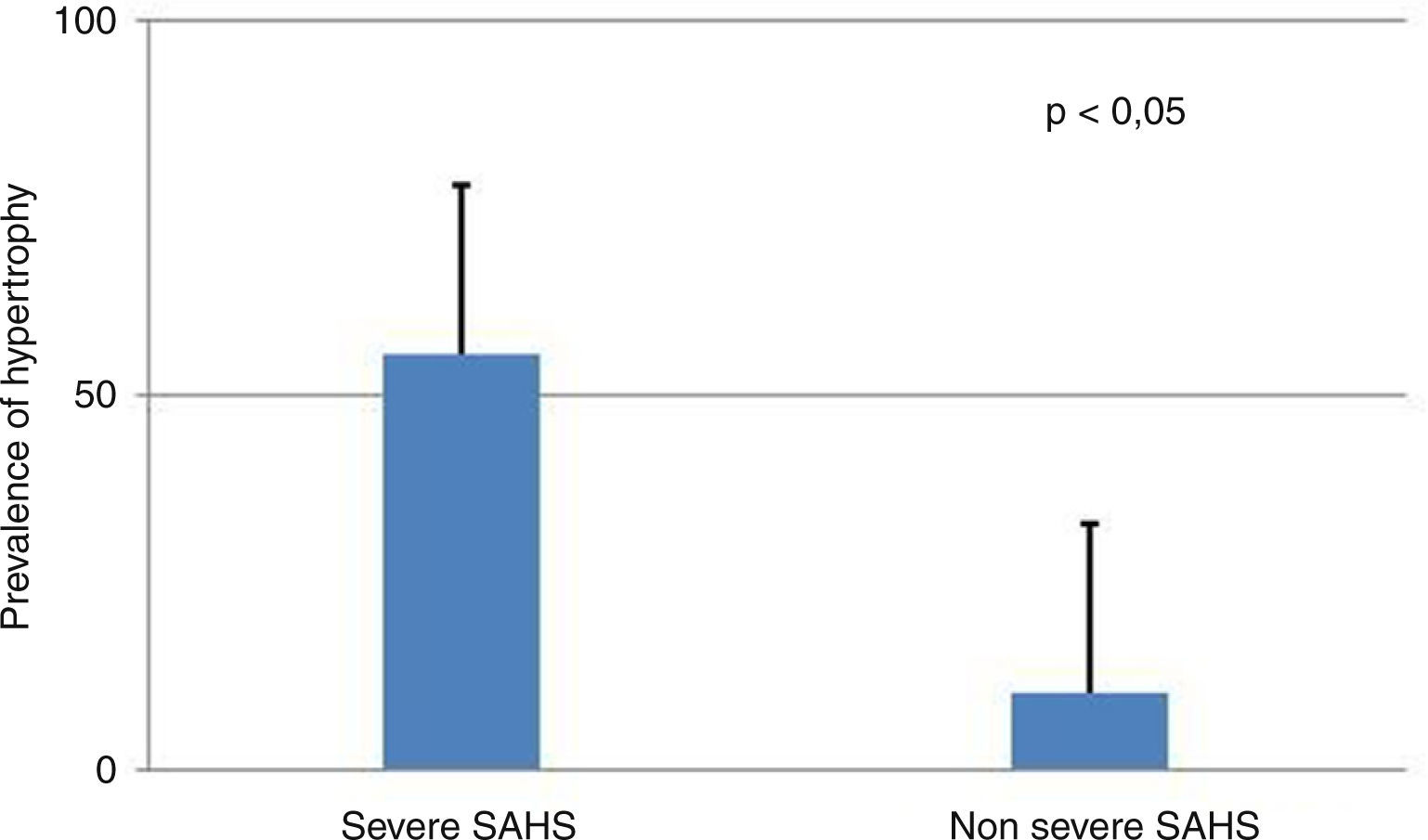
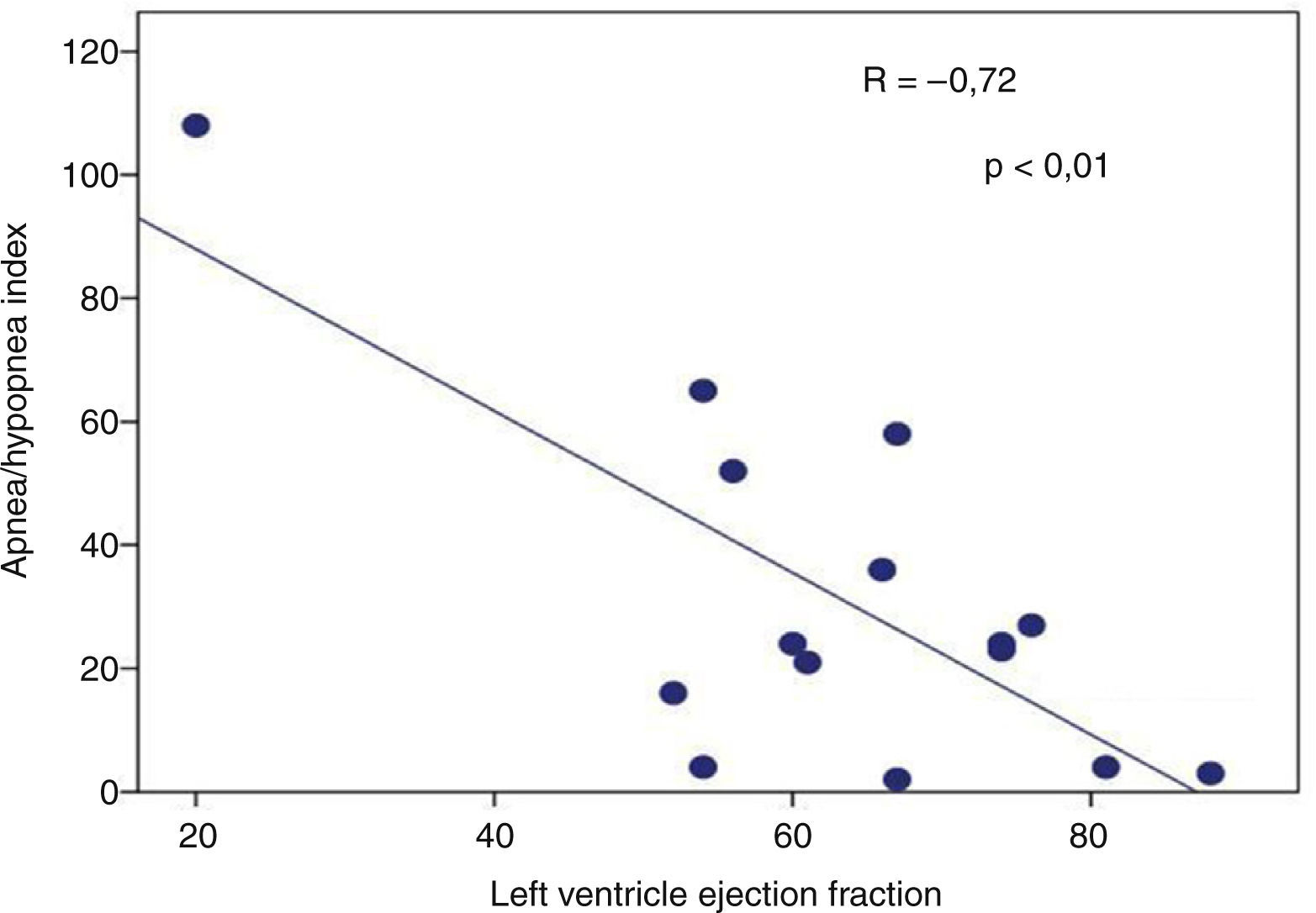
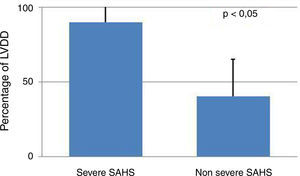
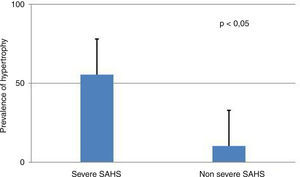
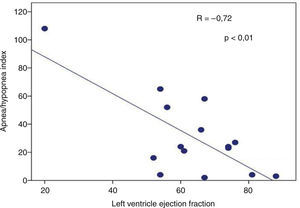
Presence of severe SAHS was related to more risk of LVDD (90.9% vs. 40%, p=0.008; OR 2.3 [1.3–4.0]) only in acromegalic group (Fig. 1). Acromegalic patients with severe SAHS also had more risk of LV hypertrophy (55.6% vs. 10.5%, p=0.02; OR 5.3 [1.3–22.2]) (Fig. 2). We did not find differences on prevalence of LV hypertrophy between acromegalic and control patients (25% vs. 35%, p=0.4%), neither between NCA and CA (38.5% vs. 16.7%, p=0.2). On multivariate analysis, only severe SAHS was related to LVDD and LV hypertrophy on acromegalic group as independent variables. Only NCA group demonstrates correlation between apnea/hypopnea index and ejection fraction LV (r=−0.72; p=0.00) (Fig. 3).
We did not find any difference on right ventricle dysfunction and acromegalic status or SAHS severity. No echocardiographic findings were related to the time of evolution of acromegaly, SSA treatment or IGF-1 levels.
Follow-upFour acromegalic patients and one control died during follow-up. One acromegalic patient was lost. On CA group, 7/13 patients were considered as cured and 3/13 maintained controlled acromegaly. After seven years, 3 patients previously well controlled undergone to uncontrolled disease. On NCA group, 3/11 patients were considered as cured, 6/11 maintained controlled acromegaly and only 2/11 remained as uncontrolled.
Acromegalic patients with severe SAHS had more risk to develop cardiac poor outcome (considered as cardiac echocardiographic alterations or ischemic cardiopathy) (p=0.013; OR 7.53. IC95%: 1.07–53.24). Patients of NCA group had a trend toward association to more ischemic cardiopathy (p=0.08; OR 2.63, IC95%: 1.52–4.53).
We did not find differences on cardiac outcome on group control between different degrees of SAHS. Only 62.5% of acromegalic patients and 66.7% of controls with severe SAHS used CPAP.
DiscussionTo the best of our knowledge this is the first study that tries to find the relationship between sleep apnea disorder and cardiac morbidity in acromegalic patients, using a control group with elevated prevalence of SAHS in order to minimize bias. The prevalence of SAHS in acromegalic patients varies between 13.2 and 97% depending on methodological characteristics of the studies.1,8,13,14 We found a high prevalence of SAHS in acromegalic group (81.3%), although the prevalence of SAHS was not different between CA and NCA group. However CA had more probability to have mild SAHS without association with disease time evolution. Roemmler et al. described also a positive correlation with disease activity but not with the duration of the disease,15 although other authors did not corroborate these findings.8,16
Acromegalic cardiomyopathy is characterized more commonly by concentric biventricular hypertrophy, associating LVDD and less frequently LV systolic dysfunction, related to disease duration.17 In our series, LVDD was more prevalent on acromegalic patients, without differences between CA and NCA groups, consistent with previous studies.7 On the other part, NCA had a trend toward more risk to develop ischemic cardiopathy during the follow up. Other authors showed improvement on cardiac function after medical therapies.8,18,19
Respect to SAHS, we found that acromegalic patients with severe SAHS had more risk to develop LV hypertrophy and LVDD, independently of duration of disease or IGF-1 status as multivariate analysis shown. Moreover, these acromegalic patients had worst cardiac outcome during the follow-up. There has been demonstrated an association of heart failure and SAHS on general population, and some studies found that SAHS treatment with positive airway ventilation could improve LV function.3,4 However, we were not able to demonstrate this association on control group; probably due to small sample size (only 3 subjects had severe sleep apnea). In contrast to our results, previous report in acromegalic cohort that was not able to associate cardiac hypertrophy and SAHS,13 although our cohorts were similar. Thus further studies should be done in order to clarify this discrepancy.
Regarding to electrocardiographycal changes, Colao et al. described higher frequency of arrhythmias in acromegalic patients than control group as we found in our study, although their study population had less prevalence in both groups (6.8 vs. 1.5%) that could be explained by the definition used for electrocardiographycal changes (our definition also considered blocks, not only arrhythmias).17 On the other hand, they did not examine association between SAHS and arrhythmias, that has been described on general population.5,20 We found electrocardiographical changes only in patients with SAHS, both in acromegalic and control group, so these changes could be explained by cardiac remodelation associated with recurrent hypoxemias and an increased sympathetic activity2,5 and we could not separate the effect of acromegaly itself.
One limitation of our study was that we did not have information about duration of medical therapy that has been associated to LVDD regression in some studies.8,18,19 About definition of acromegaly activity, we used previous cure criteria because this study was performed at 2007. We think that evaluation of cardiac rhythm could be more accurate with Holter study, but we did not perform it for logistic problems. Nonetheless, the main limitation could be the small sample size of each group that could have had insufficient statistical power.
In conclusion, SAHS is highly prevalent in acromegalic patients and it was related to electrocardiographic changes. In our series, severe SAHS in acromegalic patients associates LV hypertrophy, LV dysfunction and more cardiac events during follow-up. These results should be confirmed on larger cohort.
FundingNothing to declare.
Author contributionsNothing to declare.
Conflict of interestThe authors declare no conflict of interest.