Approximately one third of the patients with differentiated thyroid cancer (DTC) who develop structurally-evident metastatic disease are refractory to radioactive iodine (RAI). Most deaths from thyroid cancer occur in these patients. The main objective of this consensus is to address the most controversial aspects of management of these patients.
MethodsOn behalf of the Spanish Society of Endocrinology & Nutrition (SEEN) and the Spanish Group for Orphan and Infrequent Tumors (GETHI), the Spanish Task Force for Thyroid Cancer, consisting of endocrinologists and oncologists, reviewed the relevant literature and prepared a series of clinically relevant questions related to management of advanced RAI-refractory DTC.
ResultsTen clinically relevant questions were identified by the task force. In answering to these 10 questions, the task force included recommendations regarding the best definition of refractoriness; the best therapeutic options including watchful waiting, local therapies, and systemic therapy (e.g. kinase inhibitors), when sodium iodide symporter (NIS) restoration may be expected; and how recent advances in molecular biology have increased our understanding of the disease.
ConclusionsIn response to our appointment as a task force by the SEEN and GHETI, we developed a consensus to help in clinical management of patients with advanced RAI-refractory DTC. We think that this consensus will provide helpful and current recommendations that will help patients with this disorder to get optimal medical care.
Alrededor de un tercio de los pacientes con cáncer diferenciado de tiroides (CDT) que desarrollan enfermedad metastásica estructural son refractarios al yodo radiactivo. Desafortunadamente, la mayoría de las muertes debidas al cáncer de tiroides ocurren en pacientes con CDT avanzado refractario al yodo radiactivo. El principal objetivo de este consenso es abordar los aspectos más controvertidos del manejo de estos pacientes.
MétodosEn nombre de la Sociedad Española de Endocrinología y Nutrición (SEEN) y del Grupo Español de Tumores Raros e Infrecuentes (GETHI), el grupo de trabajo para el Cáncer de Tiroides, compuesto por endocrinólogos y oncólogos, revisó la literatura más destacada y desarrolló una serie de preguntas clínicamente relevantes concernientes al manejo de los pacientes con CDT refractario.
ResultadosDiez preguntas clínicamente relevantes fueron identificadas por el grupo de trabajo. En las respuestas el grupo incluyó recomendaciones sobre la mejor definición de la refractariedad, las mejores opciones terapéuticas, entre las cuales se incluyen la actitud expectante, las terapias locales y la terapia sistémica (por ejemplo inhibidores de tirosín-cinasa), cuándo esperar la recaptación de yodo radiactivo mediada por NIS y cómo los recientes avances en genética molecular han ayudado a comprender mejor la enfermedad.
ConclusiónEn respuesta a nuestro compromiso como grupo de trabajo de la SEEN y GETHI hemos creado un consenso para asistir al manejo clínico de los pacientes con CDT avanzado refractario al yodo radiactivo. Pensamos que este consenso proporcionará unas recomendaciones útiles y actualizadas que ayuden a los pacientes con esta enfermedad a tener un cuidado óptimo.
Thyroid cancer is the most prevalent type of endocrine malignancy and its incidence has been steadily increasing over the last three decades.1 Due to this rise in its incidence, thyroid cancer is currently the fifth most common new cancer diagnosis in women and the eighth most common new cancer diagnosis overall in the United States of America (USA).2 It is now more frequently diagnosed than all leukaemias combined, as well as ovarian, uterine, pancreatic, or oesophageal cancers. For the majority of patients with thyroid cancer, treatment with surgery, radioactive iodine (RAI) ablation and TSH suppressive therapy allows an overall survival (OS) rate of 97.7% at five years.3 Nevertheless, locoregional recurrence occurs in up to 20% of patients, and distant metastases in approximately 10% at 10 years. Some of these patients with locorregional recurrences and/or distant metastases lose the ability for iodine uptake leading to RAI-refractory metastatic disease. Patients with RAI-refractory metastatic disease have an overall survival rate of less than 50% at three years and account for more deaths in the USA at present than Hodgkin's lymphoma, osteosarcoma, or testicular cancer.
Differentiated thyroid cancer (DTC) derive from thyroid follicular cells and accounts for more than 90% of all thyroid cancers. The dominant histotypes are papillary and follicular cancers, while Hürthle cell thyroid cancer (a follicular thyroid cancer subtype) and poorly differentiated thyroid cancer are less common variants. Undifferentiated or anaplastic thyroid carcinomas, always RAI-refractory, are not reviewed in this consensus. Around one third of DTC patients with structurally-evident locorregional and/or metastatic disease becomes RAI-refractory, with inadequate radiation doses to malignant cells and failure to eradicate metastasis. Thus, RAI-refractory DTC is defined more by behavior than specific histopathology. Notably, although anaplastic thyroid cancers have higher mortality rates than DTC, most of the estimated deaths from thyroid cancer will be in patients with RAI-refractory DTC.2
Over the last decade, there have been substantial advances in the management of RAI-refractory DCT. Because controversy exists in some areas, the Spanish Task Force for Thyroid Cancer on behalf of Spanish Society of Endocrinology Thyroid Cancer Working Group (GTSEEN) and the Spanish Group for Orphan and Infrequent Tumors (GETHI) have created together a national task force in order to establish a consensus addressing the most challenging aspects regarding the management of these patients. To address these aspects, we developed a series of clinically relevant questions which were as follows:
- (1)
Do we really know the incidence and prevalence of RAI-refractory DTC in Spain?
- (2)
Which is the best definition of RAI-refractory DTC?
- (3)
Which patients with RAI-refractory metastatic DTC can be followed without additional therapy (watchful waiting)?
- (4)
Which patients with RAI-refractory DTC are candidate for local therapies?
- (5)
Which patients should be considered for kinase inhibitor therapy in RAI-refractory DTC patients? What kinase inhibitors do we use?
- (6)
Is sodium iodide symporter (NIS) restoration and subsequent RAI reinduction a feasible strategy in RAI-refractory DTC patients?
- (7)
How can we use the advances in molecular biology knowledge of DTC for the treatment of RAI-refractory disease?
- (8)
What is the role of chemotherapy and radiotherapy in RAI-refractory DTC?
- (9)
Is thyroglobulin a useful biomarker in RAI-refractory DTC?
- (10)
Is TSH suppression still necessary in RAI-refractory setting?
The lack of national registries for thyroid cancer patients in Spain makes difficult an estimation of the new cases per year and the percentage of patients that become refractory to RAI therapy. As a Western Europe country, Spain is expected to have an age-standardized rate of around 6 per 100,000 females.4 Available data of estimated incidence in Spain shows an incidence rate of 2.12 per 100,000 males and 6 per 100,000 females in 2004.5 If we apply the European incidence rates of new diagnoses, around 2000–2500 new cases of thyroid cancer should be diagnosed in Spain per year. If 90% of all thyroid cancers are DTCs, and 10% of them become RAI-resistant, approximately 180–200 new patients per year could be diagnosed of refractory DTC in Spain.
(2) Which is the best definition of radioactive iodine-refractory differentiated thyroid cancer?Various terms such as “refractory,” “resistant,” “nonresponsive,” or “non-avid” have been used to characterize DTC patients with locoregional and/or distant metastases for whom RAI therapy provides no further clinical benefit. The first three terms all imply that RAI therapy has not yield a clinical benefit despite cells may be avid for RAI. The last term “non-avid” describes tumors not absorbing any amount of RAI on a diagnostic or post-therapy scintigraphy. Therefore, tumors may retain their “avidity” for RAI and yet do not receive a sufficient radiation dose from therapy to yield a clinical benefit.
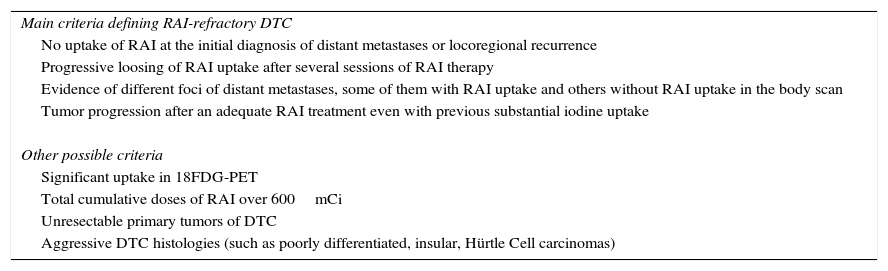
In routine clinical practice, patients with appropriate TSH stimulation and iodine preparation can be defined as RAI-refractory if they meet any of the following criteria (Table 1): (a) no uptake of RAI at the initial diagnosis of distant metastases or locoregional recurrence. (b) Progressive loosing of RAI uptake after several sessions of RAI therapy. (c) Evidence of different foci of distant metastases, some of them with uptake and some others without RAI uptake in the body scan. (d) Tumor progression after an adequate RAI treatment even with previous substantial RAI uptake. When a patient with DTC is classified as refractory to RAI, there is no indication for further RAI treatment.
Clinical criteria defining radioactive iodine-refractory differentiated thyroid cancer.
| Main criteria defining RAI-refractory DTC |
| No uptake of RAI at the initial diagnosis of distant metastases or locoregional recurrence |
| Progressive loosing of RAI uptake after several sessions of RAI therapy |
| Evidence of different foci of distant metastases, some of them with RAI uptake and others without RAI uptake in the body scan |
| Tumor progression after an adequate RAI treatment even with previous substantial iodine uptake |
| Other possible criteria |
| Significant uptake in 18FDG-PET |
| Total cumulative doses of RAI over 600mCi |
| Unresectable primary tumors of DTC |
| Aggressive DTC histologies (such as poorly differentiated, insular, Hürtle Cell carcinomas) |
RAI, radioactive iodine; DTC, differentiated thyroid cancer; 18FDG-PET, 2-deoxy18-fluoro-d-glucose positron emission tomography.
Although general agreement can be found with the previous criteria, there are other situations and definitions less clear and a consensus within multidisciplinary teams are needed before considering RAI-refractory disease and potential candidates for systemic therapy (Table 1): (a) total cumulative doses of RAI over 600mCi; (b) significant uptake in 2-deoxy18-fluoro-d-glucose positron emission tomography (18FDG-PET) integrated with computed tomography (CT). (c) Unresectable primary tumor of thyroid cancers should be managed as RAI-refractory DTCs as RAI therapy is not feasible (this status could change if tumor reduction is reached and thyroid could be removed). (d) Aggressive DTC histologies (such as poorly differentiated, insular, Hürtle cell carcinomas).
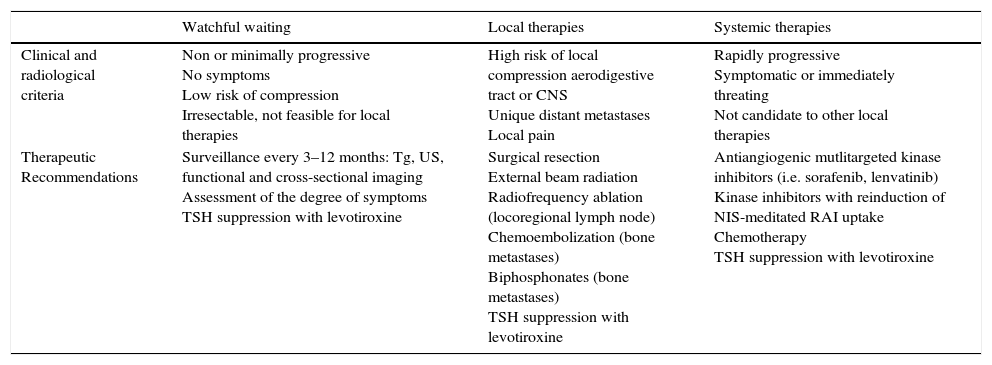
(3) Which patients with radioactive iodine-refractory metastatic differentiated thyroid cancer can be followed without additional therapy (watchful waiting)?RAI-refractory metastatic DTC can be asymptomatically stable for long periods of time and in such patients the benefits of novel therapies might be largely outweighed by drug toxicities. Therefore, there is a great risk of overtreatment if we do not discriminate appropriately these patients and anticipate the natural course of the disease. Patients with an indolent clinical course with no apparent tumor progression documented by radiological techniques, no symptoms and no adverse impact from their disease burden despite having a structurally evident metastatic disease, watchful waiting should be considered (Table 2).
Therapeutic options in radioactive iodine-refractory differentiated thyroid cancer.
| Watchful waiting | Local therapies | Systemic therapies | |
|---|---|---|---|
| Clinical and radiological criteria | Non or minimally progressive No symptoms Low risk of compression Irresectable, not feasible for local therapies | High risk of local compression aerodigestive tract or CNS Unique distant metastases Local pain | Rapidly progressive Symptomatic or immediately threating Not candidate to other local therapies |
| Therapeutic Recommendations | Surveillance every 3–12 months: Tg, US, functional and cross-sectional imaging Assessment of the degree of symptoms TSH suppression with levotiroxine | Surgical resection External beam radiation Radiofrequency ablation (locoregional lymph node) Chemoembolization (bone metastases) Biphosphonates (bone metastases) TSH suppression with levotiroxine | Antiangiogenic mutlitargeted kinase inhibitors (i.e. sorafenib, lenvatinib) Kinase inhibitors with reinduction of NIS-meditated RAI uptake Chemotherapy TSH suppression with levotiroxine |
Tg, thyroglobulin; US, ultrasonography; CNS, central nervous system; RAI, radioactive iodine; NIS, sodium iodide symporter.
There are typically three clinical situations in which watchful waiting may be an option and/or there is not sufficient evidence for more aggressive therapies. One is the presence of asymptomatic small (lower than 5–8mm) metastatic lymph nodes after RAI ablation, particularly after prior neck compartmental dissection. In these cases surgery or other local therapies have not proved any benefit in terms of improving gross clinical disease recurrences or disease-specific survival. Secondly, for some patients with small pulmonary metastatic disease (usually lower than 1cm), in which the disease is not progressing or is slowly progressing, patients can often be followed conservatively on TSH suppressive therapy. The third clinical situation in which watchful waiting is recommended is the presence of bone metastases that are asymptomatic, stable lesions that do not threaten nearby critical structures.
However, despite the indolent course of these patients, careful and thoughtful surveillance must be performed in the setting of a multidisciplinary team. Apart from determining thyroglobulin levels and neck ultrasound, attention should be directed toward determining the extent of metastatic disease by functional and cross-sectional imaging (FDG-PET, magnetic resonance (MRI), CT) and the rate of progression of radiological evident lesions by applying response evaluation criteria in solid tumors (RECIST). However, defining disease progression by RECIST when RAI-refractoriness is reached can be challenging. Often, RAI-refractory status is defined when an 18FDG-PET is performed after detection of thyroglobulin increase and negative RAI body scan. In this situation, disease progression status by RECIST is difficult to define, as no previous comparable images are usually available. In addition to imaging, assessing the degree of current or potential symptoms and understanding the co-morbidities that might influence the choice of therapy may be really helpful to make important decisions during the follow up of these patients.
Overall, patients with refractory disease that are asymptomatic, low tumor burden, stable or minimally progressive, and not likely to develop rapidly progressive disease, do not need any further treatment but TSH suppression and active surveillance. Clinically significant complications can be monitored with imaging every three to six months along with the assessment of the degree of current or potential symptoms.
(4) Which patients with radioactive iodine-refractory differentiated thyroid cancer are candidate for local therapies?The indication of local therapies for RAI-refractory DTC will be conditioned by the location and number of metastasis, tumor burden and technically feasible approaches. Locorregional recurrences are the most frequent sites of tumor relapse in DTC patients, followed by lung, extracervical lymph nodes, bones and brain. Local therapies include salvage locoregional or distant metastatic surgery, external beam radiation or radiosurgery, radiofrequency or ethanol ablation, and chemoembolization, in order to prevent complications in vital structures, such as central nervous system or central neck compartment.6 As a general rule, surgery or local therapies should be taken into account when respiratory and/or digestive tract are involved and/or at risk of failure to try to avoid locoregional complications (Table 2). A small fraction of patients may benefit from radiofrequency ablation,7 ethanol ablation,8 or embolization.9
In the case of locorregional disease, surgery is favored in the presence of bulky disease or disease amenable to surgery found on anatomic imaging such as ultrasonography, CT scanning, or MRI. In fact, surgery is the first option even if RAI uptake is present. Therapeutic compartmental central and/or lateral neck dissection, sparing uninvolved vital structures, should be performed. More limited surgery is reasonable in the case of patients who have undergone prior comprehensive neck dissection and/or external beam radiotherapy. On the other hand, it is not clear that treatment of locoregional disease is beneficial in the setting of untreatable distant metastases, except for possible palliation of symptoms or prevention of airway or aerodigestive obstruction. For tumors that invade the upper aerodigestive tract, surgery combined with external beam radiation is generally advised. Techniques ranging from shaving tumor off the trachea or esophagus for superficial invasion, to more aggressive techniques when the trachea is more deeply invaded (e.g. tracheal resection and anastomosis or laryngopharyngoesophagectomy) can be considered.
For selected patients with pulmonary metastases, local therapies need to be considered, such as metastasectomy, endobronchial laser ablation, or external beam radiation for palliation of symptomatic intrathoracic lesions (e.g. obstructing or bleeding endobronchial masses), and pleural or pericardial drainage for symptomatic effusions.
Although some bone metastases are RAI avid, RAI therapy is rarely curative in the long term and patients will need further interventions. Complete surgical resection of isolated symptomatic metastases has been associated with improved survival and should be considered, especially in patients younger than 45 years old with slowly progressive disease. For skeletal metastases, surgical palliation is recommended for symptomatic or asymptomatic tumors in weight-bearing extremities. When skeletal metastatic lesions arise in locations where acute swelling may produce severe pain, fracture, or neurologic complications, external radiation and the concomitant use of glucocorticoids to minimize potential TSH-induced and/or radiation-related tumor expansion should be strongly considered. Intravenous bisphosphonate (pamidronate or zoledronic acid) therapy may be considered for symptomatic bone metastases, and embolization of metastases can also be considered.9
(5) Which patients should be considered for multikinase inhibitor therapy in radioactive iodine-refractory differentiated thyroid cancer patients? What kinase inhibitor should we use?Currently, there is general agreement that multikinase inhibitors (MKI) therapy should be considered only in RAI-refractory DTC patients with progressive and/or symptomatic metastatic disease not otherwise amenable to local therapies. The reasons for such limitations arise from clinical trials. Since the first multicenter therapeutic trial of a MKI was performed in progressive DTC, evidence favored the clinical use of MKIs but also revealed limitations concerning drug toxicity and patient eligibility.
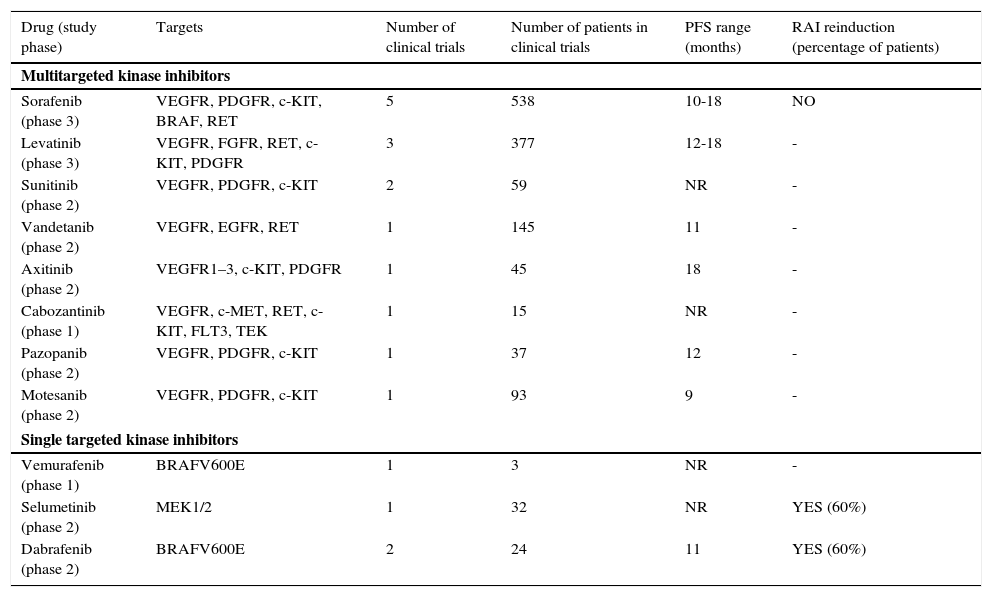
Multiple kinase inhibitors and other targeted agents have prominently changed the management of patients with advanced RAI-refractory DTC (Table 3). These drugs, mainly multikinase inhibitors with antiangiogenic effect, have now been assessed in phase 1–3 trials, and some of them have been associated with partial response and stable disease in more than 50% of treated patients. Of note, prolongation of progression free survival has been shown with sorafenib and lenvatinib compared with placebo in the two phase 3 trials published so far.10,11 These two drugs have been approved by Food and Drug Administration and European Medicines Agency for use in patients with RAI-refractory metastatic disease. Based on published phase 2 trials, other orally available multikinase inhibitors such as sunitinib, axitinib, cabozantinib or pazopanib may produce some clinical benefit in this setting but only the results of the phase III study with vandetanib are expected in the near future.12
Results of clinical trials of targeted therapies in radioactive iodine-refractory differentiated thyroid cancer.
| Drug (study phase) | Targets | Number of clinical trials | Number of patients in clinical trials | PFS range (months) | RAI reinduction (percentage of patients) |
|---|---|---|---|---|---|
| Multitargeted kinase inhibitors | |||||
| Sorafenib (phase 3) | VEGFR, PDGFR, c-KIT, BRAF, RET | 5 | 538 | 10-18 | NO |
| Levatinib (phase 3) | VEGFR, FGFR, RET, c-KIT, PDGFR | 3 | 377 | 12-18 | - |
| Sunitinib (phase 2) | VEGFR, PDGFR, c-KIT | 2 | 59 | NR | - |
| Vandetanib (phase 2) | VEGFR, EGFR, RET | 1 | 145 | 11 | - |
| Axitinib (phase 2) | VEGFR1–3, c-KIT, PDGFR | 1 | 45 | 18 | - |
| Cabozantinib (phase 1) | VEGFR, c-MET, RET, c-KIT, FLT3, TEK | 1 | 15 | NR | - |
| Pazopanib (phase 2) | VEGFR, PDGFR, c-KIT | 1 | 37 | 12 | - |
| Motesanib (phase 2) | VEGFR, PDGFR, c-KIT | 1 | 93 | 9 | - |
| Single targeted kinase inhibitors | |||||
| Vemurafenib (phase 1) | BRAFV600E | 1 | 3 | NR | - |
| Selumetinib (phase 2) | MEK1/2 | 1 | 32 | NR | YES (60%) |
| Dabrafenib (phase 2) | BRAFV600E | 2 | 24 | 11 | YES (60%) |
PFS: progression free survival; RAI: radioactive iodide. BRAF: B-Raf proto-oncogene, serine/threonine kinase. VEGFR: Vascular endothelial growth factor receptor; PDGFR: platelet-derived growth factor receptor; FGFR: fibroblast growth factor receptor; KIT: proto-oncogene c-kit; RET: ret proto-oncogene; EGFR: epidermal growth factor receptor; c-MET: proto-oncogene that encodes hepatocyte growth factor receptor; FLT3: fms-related tyrosine kinase 3. TEK: tyrosine kinase, endothelial; MEK1/2: mitogen-activated protein kinase kinase ½.
Despite these promising results, there are some limitations on the use of MKI in advanced RAI-refractory DTC. The most important one is patient's selection as no biological or genetic biomarker has been yet elucidated to predict tumor response or patient's outcome. The indolent course of the disease that can be observed in some cases with low tumor burden can jeopardize the efficacy of an early initiation of therapy and increase the risk of toxicity. However, waiting for high tumor burden or for the onset of symptoms could compromise the survival of patients.10 Additionally, some complete responses are started to be seen with MKI in this setting as well as trends in increasing overall survival in some subgroup analyses even with the crossover design of the placebo-controlled clinical trials suggesting the capacity to change the natural history of this disease.11,13
In addition, drug-related side effects are common and could reduce patients’ quality of life and increase the risk of drug-related deaths. The optimal management of these risks is clinically relevant, especially in view of the necessity of long-term application of these treatments in most cases. Most common side effects observed with MKI include fatigue, hand-foot syndrome, hypertension, diarrhea, skin rashes and erythema, weight loss, and various drug-specific toxicities that have been reported. These side effects, although often mild and responsive to supportive care measures, may prominently reduce patients’ quality of life and treatment with these agents should be limited to specialists experienced in their use.
In summary, the use of MKI therapy should, therefore, be limited to patients with a progressive, metastatic and clinically relevant tumor burden disease that is not amenable to palliation with surgery or locoregional approaches. Patients with advanced progressive RAI-refractory DTC should additionally be referred to experienced centers that can offer interdisciplinary expertise in the individualization of treatment and participation in therapeutic clinical trials.
(6) Is sodium iodide symporter (NIS) restoration and subsequent radioactive iodine reinduction a feasible strategy in radioactive iodine-refractory differentiated thyroid cancer patients?Advanced RAI-refractory DTC shows negligible 131I uptake due to loss of functional sodium iodide symporter SLC5A5 expression. As long as a few NIS molecules are functionally expressed in thyroid cancer cells, thyroid cancer can be successfully treated with RAI administration. RAI selectively targets and destroys any remnant or metastatic NIS-expressing thyroid cancer cells. Decreased expression of NIS and/or impaired targeting of NIS to the membrane of thyroid cancer cells (which is required for NIS functional activity), are the mechanisms underlying RAI-refractory disease. Cloning of the NIS gene and its characterization have allowed investigation of the molecular mechanisms leading to NIS loss of function, with potential to provide novel approaches for DTC therapy, intending to restore NIS-mediated iodide accumulation.
NIS is a key target for novel thyroid cancer therapies. Although traditional therapies (e.g. retinoic acid) have shown some reinduction in RAI, such therapies have never been sufficient to reach a therapeutic effect.12 Notably, novel therapies, particularly single kinase inhibitors, have paved the way to significant RAI reinduction (Table 3). The MEK inhibitor selumetinib has been the first agent to reinduce iodide uptake in sufficient amount to exert a RAI-induced cytotoxic effect.14 In a prospective clinical trial with 20 patients with RAI-refractory DTC, 12 patients showed a significant RAI uptake, and eight of them had a partial response. Therapeutic efficacy seemed to be significantly prominent in RAS mutated tumors as all of five mutant tumors showed increased RAI uptake, with four demonstrating partial response and one stable disease. Only one patient out of nine with a BRAFV600E positive tumor showed significant RAI uptake, but RAI therapy had a particularly striking effect. Dabrafenib, a selective inhibitor of mutant BRAF, has been the second agent to show promising results.15 Six of 10 patients (60%) with BRAFV600E mutant RAI-refractory papillary thyroid carcinoma (PTC) showed a significant RAI uptake and two of them had a partial response and four patients had stable disease. A potential advantage of this therapeutic strategy (compared with long-term treatment with kinase inhibitors) is that a short course of kinase inhibitor therapy (45 days) is required, which could limit side effects. However, there are also some limitations with this approach, including the risk of hematological toxicity with accumulative doses of RAI therapy and the uncertainty in the relation of the reinduction of uptake of iodine and long-term responses. Two international phase II and III studies are currently evaluating the efficacy of selumetinib in RAI sensitive metastatic patients and also in adjuvant setting.
(7) How can we use the advances in molecular biology knowledge of differentiated thyroid cancer for the treatment of radioactive iodine-refractory disease?The deeper knowledge of the main molecular steps that lead to the transformation of the normal follicular cell into an invasive thyroid carcinoma has developed several translational and clinical studies that have opened a more optimistic point of view in the treatment of these patients. Thyroid carcinogenesis has become one of the most fascinating models and a particularly promising paradigm for targeted therapy. A multistep model involving the main genetic changes that carry out the tumor initiating process of thyroid cancer of follicular cell origin has been suggested and also the so called oncogenic addiction to these particular mutations that make them particularly interesting for directed therapy.
One of the most important activating genetic aberrations in the signal transduction pathways of thyroid cancer development involves the RET (rearranged during transfection)/PTC-RAS-RAF-MAPK axis, with mutations in protein kinase RAS and BRAF kinase (BRAF) in more than half of the patients with DTC, that added to the proangiogenic environment described in advanced thyroid cancer have facilitated the development of multikinase inhibitors directed to angiogenic and lymphangiogenic-related tyrosine kinases, BRAF and RET proteins showing significant results in this setting.16 Specifically, sorafenib, a RET, BRAF, vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR) inhibitor, and lenvatinib, a RET, VEGFR, PDGFR and fibroblast growth factor receptor inhibitor, have demonstrated a significant impact increasing the median progression-free survival of patients with advanced RAI-refractory DTC.
The PI3K-AKT-mTOR pathway, thought to be a major oncogenic driver of follicular thyroid carcinomas (FTC) and the follicular variant of PTC, is also facilitating the development of kinase inhibitors. Mutations of RAS, the most frequent genetic abnormalities in FTC, are dual activators as they can stimulate both the PI3K-AKTmTOR and the MAPK pathway. The former pathway is also activated by inactivating mutations in the tumor suppression gene PTEN, or by activating mutations in the PI3KCA and AKT1, which are common in follicular thyroid carcinoma, particularly in the most aggressive forms, and in poorly differentiated and anaplastic carcinomas. Moreover, simultaneous activation of both pathways becomes more frequent in advanced RAI-refractory DTC with metastatic disease. Because of activation of the PI3K-AKT-mTOR pathway, phase 2 trials of mTOR inhibitors (everolimus and temsirolimus), alone or in combination with MKIs, are underway in patients with advanced RAI-refractory DTC.17,18
Although the advances in molecular biology of thyroid cancer pathogenesis and the development of targeted therapies in this setting have been outstanding during the last years, nowadays no predictive biomarkers of clinical benefit for these targeted agents are available. Nevertheless, genotyping for the main genetics events in refractory DTC (BRAF, RAS, RET/PTC, etc.) may be considered in patients with advanced RAI-refractory DTC since it may help in the future to better understand the mechanisms underlying the responsiveness and resistance to novel therapies such as kinase inhibitors.
(8) What is the role of chemotherapy and radiotherapy in radioactive iodine-refractory differentiated thyroid cancer?Classical chemotherapeutic agents have shown limited activity in DTC. There are several trials that have evaluated the activity of different agents in patients with DTC with disappointing results, between 0 and 20% of response rates, of short duration, without complete remissions and no impact in overall survival. The most developed drug has been doxorubicin alone and in combination with other cytotoxics, mainly cisplatin, with no increasing in response rates but higher adverse events. Doxorrubicin is the only chemotherapeutic agent approved for refractory DTC and current recommendations limit its use, alone or in combination with taxane or platinum-based chemotherapy in anaplastic thyroid carcinoma.19
External bean radiotherapy has been used to improve the locoregional disease control and to palliate some locations of distant metastases, such as central nervous system or bones. However, current recommendations for locoregional disease control are limited to macroscopic unresectable tumors in older patients and in combination with radiosensitizer drugs in anaplastic thyroid cancer.20
(9) Is thyroglobulin a useful biomarker in radioactive iodine-refractory differentiated thyroid cancer?Thyroglobulin serum levels have been one of the most important tools to follow up patients with persistent or recurrent disease. Usually, significant increasing in thyroglobulin levels, particularly a thyroglobulin doubling time less than one year, suggests disease progression. In this situation, successive sessions of RAI therapy until the tumor is cured or becomes resistant to RAI therapy are recommended. When refractoriness appears, thyroglobulin levels could lose accuracy in correlation with tumor burden in parallel with the dedifferentiation process of DTC. Additionally, no clear correlation has been established with systemic therapies and thyroglobulin levels.
For these reasons, no systemic therapies should be started based only in thyroglobulin levels, as well as no systemic therapies should be stopped based on thyroglobulin variations.
(10) Is TSH suppression still necessary in radioactive iodine-refractory setting?Overall TSH suppression is suggested in patients with DTC after surgery to reduce the risk of recurrence.21 Initial suppression below 0.1mU/L is recommended in high and intermediate risk thyroid cancer patients, while maintenance below lower normal limit (0.5mU/L) is appropriate for low risk patients. However, no clear recommendation is available TSH suppression in RAI-refractory DTC. The progressive undifferentiating process that DTC cells suffer during the disease period time produces a TSH-independent growth that calls into questions TSH suppression in this setting. Additionally, most new targeted agents produce some refractoriness to levothyroxine and TSH increase is commonly seen in thyroid cancer patients treated with MKIs being necessary an increase of levothyroxine doses to maintain TSH levels within normal range and to prevent a greater fatigue syndrome typically observed in these patients.
Concluding remarks. Future directions and researchMultidisciplinary management of thyroid cancer should be mandatory and focused in referral centers to maximize patients’ chances for being cured. Future research will be focused in the search of prognostic biomarkers and better patients’ molecular characterization, redifferentiation process of follicular thyroid cancer cells leading to NIS restoration and RAI reinduction, optimization and innovation in nuclear medicine targeted therapy and novel molecular targeted agents and combinations that are currently changing the natural history of RAI-refractory DTC.
Conflict of interestsGarcilaso Riesco-Eizaguirre has received honoraria for advisory boards or lectures from Genzyme, Merck and Bayer.
Juan Carlos Galofré has been a consultant for AstraZeneca, Bayer, and Genzyme, and has received speaker honoraria from Genzyme and Merck.
Enrique Grande: advisory/speaker role of Bayer y Eisai.
Jaume Capdevila: advisory/speaker role of Bayer and Eisai, and has received research funding from Bayer.
Elena Navarro González: advisory/speaker role of Bayer.
Javier Santamaría Sandi has received speaker honoraria from Genzyme.
Please cite this article as: Riesco-Eizaguirre G, Galofré JC, Grande E, Zafón Llopis C, Ramón y Cajal Asensio T, Navarro González E, et al. Consenso español sobre el tratamiento de los pacientes con cáncer de tiroides diferenciado avanzado resistente al yodo radiactivo. Endocrinol Nutr. 2016;63:e17–e24.