The launching of the Precision Medicine Initiative by the President of the United States in January 2015 was an invitation for all healthcare professionals to review their practice. This call should stimulate thyroidologists working in different areas (from basic research or epidemiology to the frontline of the clinical arena or to those designing public health programs) to be aware of this new outlook. The aim of the initiative is to eradicate imprecision in estimating the probability of a correct diagnosis, to be as sure as possible of the most effective treatment, and to maximize the chances of a successful outcome. This paper summarizes some of the current challenges faced by endocrinologists in the field of thyroid dysfunction, and illustrates how precision medicine may improve diagnosis and therapy in the future.
El lanzamiento de la iniciativa medicina de precisión (Precision Medicine Initiative), en enero de 2015, por el Presidente de los Estados Unidos, ha supuesto una invitación a todos los profesionales sanitarios para revisar su modo de actuar. Esta llamada debe estimular a los tiroidólogos que trabajan en todos los campos (desde la investigación básica o la epidemiología, hasta aquellos que se encuentran en la primera línea del quehacer clínico o los que diseñan programas de salud pública), para estar atentos a este nuevo panorama. El objetivo de la iniciativa es erradicar la imprecisión a la hora de estimar la probabilidad de un diagnóstico correcto o tener la mayor certeza posible sobre la terapia más eficaz, y ampliar las posibilidades de un resultado exitoso. Este trabajo resume algunos de los desafíos actuales con los que nos enfrentamos los endocrinólogos en el campo de la disfunción tiroidea, e ilustra modos de cómo la medicina de precisión puede mejorar el diagnóstico y el tratamiento en el futuro.
History shows that there has been continuous progress in medical science over the centuries. In recent years, a number of factors have led to the pace of this knowledge to increase from arithmetical to geometrical proportions. The list of explanatory factors is broad, including more accurate clinical assessments, a better understanding of cellular reactions and environmental influences. The more precise methods for molecular characterization of patients such as the continuous increasing number of ‘omics’: (proteomics, metabolomics, genomics, etc.), together with the integration of all science, the rapid interchange of knowledge among researchers, the immense possibilities of informatics in the analysis of large databases, and the growing world of robotics and artificial intelligence are among the major factors for this remarkable progress.
However, despite the availability of all these marvelous tools, the approach to prevention, diagnosis and treatment of many conditions is currently still very often based on probabilities. Frequently we offer our patients an estimation of the chance to be diagnosed or cured from a particular disease. Our knowledge of the biological variability (due to many known and even more unknown influences) still prevents us to be more accurate and precise.
In this scenario, the launching of the Precision Medicine Initiative by the President of the United States in January 2015 has come just at the right moment.1 The Initiative has two main components to help to eradicate imprecision, with a near term focus on cancer, and a longer term focus on healthcare in general. These aims will permit better evaluation of disease risk, an understanding of their mechanisms, and prediction of the best treatments. Precision medicine has been defined as “treatments targeted to the needs of individual patients on the basis of genetic, biomarker, phenotypic, or psychosocial characteristics that distinguish a given patient from other patients with similar clinical presentations”.2 But, as a whole, it covers a much broader spectrum.
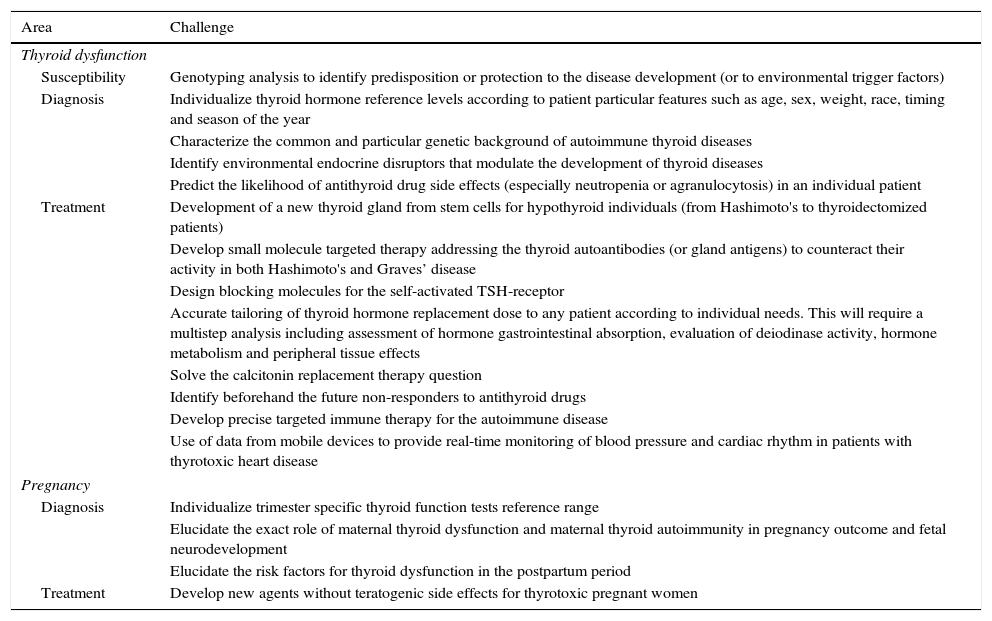
The benefits of precision medicine should apply to all human disease. Although most thyroid diseases are usually easily diagnosed and fully treatable, there are still ways in which the thyroid health of our patients can be improved. Table 1 summarizes some of the current challenges diagnosing and treating diseases related to thyroid function, excluding thyroid neoplastic disease.
Current challenges in non-tumoral thyroid diseases.
| Area | Challenge |
|---|---|
| Thyroid dysfunction | |
| Susceptibility | Genotyping analysis to identify predisposition or protection to the disease development (or to environmental trigger factors) |
| Diagnosis | Individualize thyroid hormone reference levels according to patient particular features such as age, sex, weight, race, timing and season of the year |
| Characterize the common and particular genetic background of autoimmune thyroid diseases | |
| Identify environmental endocrine disruptors that modulate the development of thyroid diseases | |
| Predict the likelihood of antithyroid drug side effects (especially neutropenia or agranulocytosis) in an individual patient | |
| Treatment | Development of a new thyroid gland from stem cells for hypothyroid individuals (from Hashimoto's to thyroidectomized patients) |
| Develop small molecule targeted therapy addressing the thyroid autoantibodies (or gland antigens) to counteract their activity in both Hashimoto's and Graves’ disease | |
| Design blocking molecules for the self-activated TSH-receptor | |
| Accurate tailoring of thyroid hormone replacement dose to any patient according to individual needs. This will require a multistep analysis including assessment of hormone gastrointestinal absorption, evaluation of deiodinase activity, hormone metabolism and peripheral tissue effects | |
| Solve the calcitonin replacement therapy question | |
| Identify beforehand the future non-responders to antithyroid drugs | |
| Develop precise targeted immune therapy for the autoimmune disease | |
| Use of data from mobile devices to provide real-time monitoring of blood pressure and cardiac rhythm in patients with thyrotoxic heart disease | |
| Pregnancy | |
| Diagnosis | Individualize trimester specific thyroid function tests reference range |
| Elucidate the exact role of maternal thyroid dysfunction and maternal thyroid autoimmunity in pregnancy outcome and fetal neurodevelopment | |
| Elucidate the risk factors for thyroid dysfunction in the postpartum period | |
| Treatment | Develop new agents without teratogenic side effects for thyrotoxic pregnant women |
The future application of precision medicine in thyroid dysfunction implies the development of new approaches for detecting, measuring, and analyzing a wide range of biomedical information. A better understanding of the genetic and environmental determinants of the diverse forms of thyroid dysfunction will improve our ability to assess disease susceptibility and identify risk factors. This improved knowledge will provide means to better diagnose thyroid dysfunction more accurately, and use more individualized targeted therapies, reducing side effects and making patient monitoring more precise.
Precision in diagnosis of thyroid dysfunctionOver the past few years there has been a considerable effort to be more accurate in the biochemical diagnosis of thyroid dysfunction. In addition to the phenotypical differences (such as age, gender, weight, race, etc.), genetic factors may be responsible, at least in part, for the variability of thyrotropin (TSH) and thyroid hormone serum concentrations.3 Some recent observations, such as the association between the nuclear factor kappa B (NFkB) and the angiotensin converting enzyme (ACE) gene polymorphisms with thyroid hormone levels,4 indicate how complex the analysis of all the causal factors in a given individual could be.
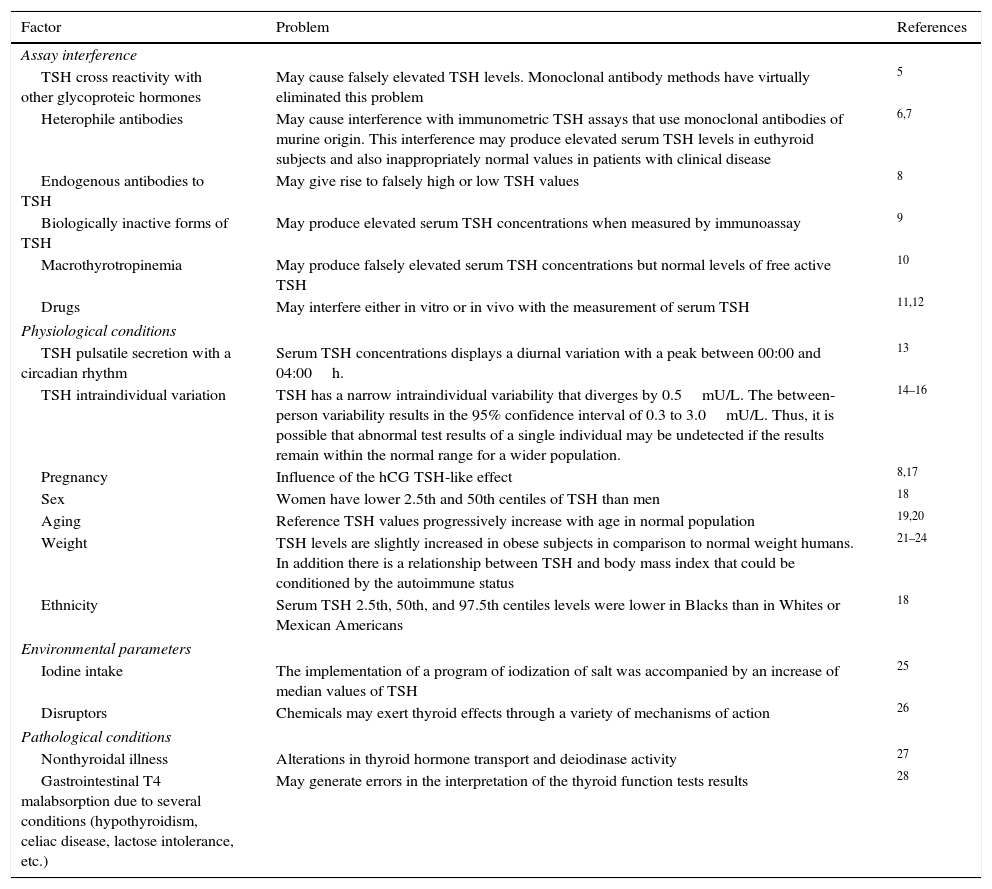
TSHSerum TSH measurement is the cornerstone in the diagnosis of thyroid dysfunction.5 Unfortunately there are a number of issues in the assessment of circulating TSH6–28 (Table 2). The numerous potential causes of assay interference illustrate the importance of a close collaboration between clinicians and the clinical chemistry laboratory staff, to avoid unnecessary testing and inappropriate treatments. Current third generation TSH assays have greatly minimized, but have not eliminated, the potential for assay interference.29,30
Factors that may cause imprecise serum TSH level assessment.
| Factor | Problem | References |
|---|---|---|
| Assay interference | ||
| TSH cross reactivity with other glycoproteic hormones | May cause falsely elevated TSH levels. Monoclonal antibody methods have virtually eliminated this problem | 5 |
| Heterophile antibodies | May cause interference with immunometric TSH assays that use monoclonal antibodies of murine origin. This interference may produce elevated serum TSH levels in euthyroid subjects and also inappropriately normal values in patients with clinical disease | 6,7 |
| Endogenous antibodies to TSH | May give rise to falsely high or low TSH values | 8 |
| Biologically inactive forms of TSH | May produce elevated serum TSH concentrations when measured by immunoassay | 9 |
| Macrothyrotropinemia | May produce falsely elevated serum TSH concentrations but normal levels of free active TSH | 10 |
| Drugs | May interfere either in vitro or in vivo with the measurement of serum TSH | 11,12 |
| Physiological conditions | ||
| TSH pulsatile secretion with a circadian rhythm | Serum TSH concentrations displays a diurnal variation with a peak between 00:00 and 04:00h. | 13 |
| TSH intraindividual variation | TSH has a narrow intraindividual variability that diverges by 0.5mU/L. The between-person variability results in the 95% confidence interval of 0.3 to 3.0mU/L. Thus, it is possible that abnormal test results of a single individual may be undetected if the results remain within the normal range for a wider population. | 14–16 |
| Pregnancy | Influence of the hCG TSH-like effect | 8,17 |
| Sex | Women have lower 2.5th and 50th centiles of TSH than men | 18 |
| Aging | Reference TSH values progressively increase with age in normal population | 19,20 |
| Weight | TSH levels are slightly increased in obese subjects in comparison to normal weight humans. In addition there is a relationship between TSH and body mass index that could be conditioned by the autoimmune status | 21–24 |
| Ethnicity | Serum TSH 2.5th, 50th, and 97.5th centiles levels were lower in Blacks than in Whites or Mexican Americans | 18 |
| Environmental parameters | ||
| Iodine intake | The implementation of a program of iodization of salt was accompanied by an increase of median values of TSH | 25 |
| Disruptors | Chemicals may exert thyroid effects through a variety of mechanisms of action | 26 |
| Pathological conditions | ||
| Nonthyroidal illness | Alterations in thyroid hormone transport and deiodinase activity | 27 |
| Gastrointestinal T4 malabsorption due to several conditions (hypothyroidism, celiac disease, lactose intolerance, etc.) | May generate errors in the interpretation of the thyroid function tests results | 28 |
A recent meta-analysis has identified some TSH-associated loci that contribute not only to TSH variation within the normal range, but also to TSH values outside the reference range that can masquerade as thyroid dysfunction. Overall, the results of this study explain, respectively, 5.6% and 2.30% of total TSH and FT4 trait variance,31 and such findings may have consequences for personalized decisions in treating hypothyroidism or hyperthyroidism. Although not in use at present, it is possible that genetic studies of TSH variances might be utilized in the interpretation of laboratory data in patients with thyroid dysfunction. However, the percentages of variance for TSH and FT4 are too low for precision medicine, indicating that we are currently too far away to be able to achieve accurate results with the present genetic information. In order to be more precise in the future, we will need additional information that takes into account fundamental knowledge of common vs. rare variants of the TSH gene, epigenetics, and gene-environment interactions.
Thyroid hormonesHighly sensitive assays are now widely available for measurement of free T4 and free T3 serum levels.6 Although not in use in clinical practice in most centers, serum concentrations of free T4 and free T3 may be measured with high precision using liquid chromatography tandem mass spectrometry (LC/MS). This new technique achievement improves assay precision, but we are still far from patient specific accuracy, because current practice is still utilizes the “one size fits all” policy, with no clear free T4 and free T3 reference range for an individual patient. In other words, we know that there exists individual variability,15 but we are currently unable to resolve this problem.
Hyperthyroidism and precision medicineDiagnostic issuesDiagnosis of Graves’ diseaseAlmost two centuries after the description of the disease by Robert Graves, we still do not know what triggers the development of this condition. By analyzing monozygotic twins, it is known that there is a genetic predisposition for developing the autoimmune disorder. But there are several environmental and epigenetic factors that influence the onset of the disease.32 Some susceptibility elements have been identified, such as particular genotypes of HLA, CTLA-4, CD40 or thyroglobulin.33 But, it has been not possible to predict the combination of genetic alterations that lead to autoimmune hyperthyroidism in a given individual. Some recent data shed light on this point: there is evidence that a epigenetic-genetic interaction involving a noncoding single nucleotide polymorphism (SNP) in the TSH receptor (TSHR) gene alters the thymic expression of this gene and has implications in triggering Graves’ disease.34
Antibody assessment in Graves’ diseaseThe presence of antibodies (Ab) against the TSHR (TRAb) is critical for the diagnosis of Graves’ disease. These antibodies could activate, inhibit or be neutral to the TSHR. Although the performance of current TRAb assays in the differential diagnosis of hyperthyroidism is excellent,35,36 some patients with hyperthyroidism and diffuse goiter have undetectable antibody levels, adding imprecision to the current diagnostic tools. On the other hand some patients with detectable TRAb have silent thyroiditis rather than Graves’ disease, as evidenced by a low radioiodine uptake, adding imprecision to the antibody based diagnosis. It is also noteworthy that some patients with Hashimoto's thyroiditis have high titer TSH receptor antibodies.
Non-autoimmune hyperthyroidismWe are far from a finely tuned diagnosis of non-autoimmune hyperthyroidism. For instance, constitutively activating germline mutations of the TSHR gene have been identified as a cause of non-autoimmune hyperthyroidism, while constitutively activating somatic mutations of the TSHR gene are detected in approximately half of toxic multinodular goiters.37 The prevalence of a TSHR mutation was found to be 4.5% in 89 hyperthyroid patients with diffuse goiter and negative TRAb in a recent study.38 This finding was accompanied by prognostic implications. The main difference in the clinical outcome of hyperthyroidism was that no patients with a TSHR mutation achieved euthyroidism over the follow-up period, while 23.5% of patients without a mutation had a remission with antithyroid drug therapy. Our current knowledge of the likelihood of remission with antithyroid drugs is thought to be higher in patients who are negative for TRAb. However, this study illustrates that some antibody negative patients may not have autoimmune thyroid disease, and that TSHR genotyping may enhance our ability to prognosticate.
Therapeutic issuesTailoring therapy to patient's diversityCurrently, the vast majority of patients with hyperthyroidism receive similar treatment for their disease, irrespective of the etiology or individual variations. Some of those therapies seem to work well for some individuals but not for all. The explanation for this difference in clinical outcomes is not clear. A precise classification of patients with hyperthyroidism that takes into account genetic, environmental and lifestyle differences would reveal why patients with an apparent identical problem differ in their responses to similar management strategies. Among many treatment options for Graves’ disease, precision medicine will allow us to use the right treatment at the right dose, given to the right patient at the right time. For instance, we should be able to identify those patients who are more likely to be resistant to, or who will relapse after a course of thionamide therapy. Ideally, physicians should know beforehand which patients may develop potentially life-threatening side effects such as agranulocytosis or hepatotoxicity. We are not far from achieving this goal. In fact, a recent genome-wide association study,39 in patients with Graves’ disease with and without agranulocytosis, has identified two loci (HLA-B*38:02 and HLA-DR *08:03) as major genetic determinants of anti-thyroid drug-induced agranulocytosis. Also, in this setting, pharmacogenomic data will facilitate the selection of a particular drug treatment and an individualized dose and dosing schedule for that medication.40 Such information may allow the early use of the most appropriate therapy (drugs, surgery or radioiodine) from the beginning, rather than wasting time or resources, as is currently the case in some clinical situations.
Molecular evaluationGenomic and metabolomic investigations will make it possible to scale up our knowledge from a few genes and metabolites to many thousands. This will enable us to better select the drug or treatment most likely to be effective with the lowest risk of adverse events. Pharmacogenomics and pharmacometabolomic studies will provide tools for mapping drug effects and for identifying pathways that contribute to higher accuracy in selecting specific therapies, as has been already tested in some diseases.41
In the near future, modification of antithyroid drug doses might be made according to the individual's genetic profile. For instance, some polymorphisms related to the transcriptional level of FOXP3 have been related to the likelihood of inducing remission of Graves’ disease.42
ImmunotherapyTargeted immunotherapy for some thyroid-related diseases, such as Graves’ ophthalmopathy will be available in the future.43 In this regard, proteomic analysis of orbital protein expression in patients with thyroid ophthalmopathy is now possible.39 Some specific proteins have been found to be up-regulated while others down-regulated in orbital fat tissue from patients with thyroid ophthalmopathy in comparison with controls. The over-expressed proteins including guanine nucleotide-binding protein, isocitrate dehydrogenase, annexin A2, heat shock protein 60, calreticulin, protein disulfide-isomerase A3, spectrin, superoxide dismutase, and transitional endoplasmic reticulum ATPase, may contribute to increased TSHR expression and cell proliferation.44 However, while proteomics may be used to design an individualized treatment strategy, there is not enough evidence to base success only on proteomics. Certainly, more information on substances such as inflammatory markers or cytokines will be important to define a successful therapy for a given individual.
New opportunities for hypothyroidismThe patient-centered approach for diagnosis and treatment of hypothyroidism is a growing field of interest for endocrinologists. Individual response to a given injury may greatly vary according to the particular genetic background. Previous exposures and simultaneous influence of several environmental factors (such as endocrine disruptors) could certainly modulate such response. The role of microchimerism in the pathogenesis of autoimmune thyroid disease has been postulated and still remains as an attractive hypothesis.45 In a similar way as noted for hyperthyroidism, precision medicine (with the help of the “omics”) will increase our ability to recognize genetic and molecular differences among individuals with hypothyroidism. Genomic factors may indicate the individual risk of developing a given particular disease or clinical course. The human phenotype may be more complex than a mere set of proteins based on coding regions of DNA. The maternal-fetal programming is a novel idea of new forms of inheritance.46
Diagnostic issuesGenetic predisposition and autoimmune factorsAntibodies against the thyroid peroxidase (TPO) play a key role in the development of Hashimoto's thyroiditis,47 the most frequent cause of hypothyroidism. Although serum TPO antibodies (TPOAb) level is a useful clinical marker for the detection of early autoimmune thyroid disease, it remains controversial if these antibodies play a causative role in the pathogenesis of Hashimoto's thyroiditis.48 Around 70% of susceptibility to develop thyroid autoantibodies is attributable to genetic factors.49 However, specific genetic factors that influence the risk of TPOAb positivity and autoimmune disease are largely unknown. This indicates that environmental and endogenous factors (such as pregnancy and immune response gene rearrangements or the presence of some particular microRNAs) could be critical in determining whether disease will develop in a predisposed individual.50 Moreover, the distribution of different autoimmune diseases within families can be explained by the inheritance of separate genes that increase susceptibility to autoimmune diseases in general (i.e., CTLA-4) and specifically to thyroid diseases (TSHR, TPO and NIS), as well as genes which may be protective.51
A recent genome wide association study (GWAS) meta-analysis for TPOAb conducted over 18,297 individuals from 11 populations shed new light on this elusive area. The main findings include significant associations for TPOAb positivity with variants at TPO, ATXN2, BACH2, MAGI3, and KALRN genes. Individuals carrying multiple risk variants also had a higher risk of increased TSH serum levels (including subclinical and overt hypothyroidism), and a decreased risk of goiter. Interestingly, the MAGI3 and BACH2 variants were associated with an increased risk of hyperthyroidism, though the MAGI3 variant was also associated with an increased risk of hypothyroidism.52 In the opinion of the authors, these results provide insight into why individuals with thyroid autoimmunity do or do not eventually develop thyroid disease. These markers may therefore predict which TPOAb-positive individuals are particularly at risk of developing clinical thyroid dysfunction. Future research will add to this information and help in the goal of more accurate prediction of systemic autoimmune disease in at risk family members of patients with autoimmune thyroid disease.
A new area of research is the role of microRNAs in the development of autoimmune thyroid disease. Serum miR-22, miR-375 and miR-451 levels are increased in the serum patients with Hashimoto's thyroiditis. In contrast, serum levels of miR-16, miR-22, miR-375 and miR-451 were increased in patients with Graves’ disease compared with healthy subjects.53 In the future, measurement of miRNA's may play an important role in diagnosing autoimmune thyroid disease.
Therapeutic issuesGenetic variability and treatment adjustmentAccurate and personalized thyroid replacement therapy continues to evolve. Although monotherapy with LT4 is the standard of care for hypothyroidism, not all patients normalize their serum T3 levels, with some experts advocating combination therapy with LT4 and liothyronine.54 Additionally, other variables modulate the response to therapy, such as the physiological circadian rhythm of serum T3, which is not replicated with the current therapy.55
As mentioned above, genetic markers that predict the risk of developing an underactive thyroid gland or a different response to therapy are not available at present. Nevertheless, some progress has been made. Inherited variations in the deiodinase 2 (D2) gene are associated with impaired baseline psychological well-being in hypothyroid patients on T4 monotherapy, and enhanced response to combination T4/T3 therapy.56 This D2 gene variation in combination with additional genetic polymorphisms in other deiodinases, (or in any molecule involved in thyroid hormone metabolism, such as thyroid hormone transporters), could very well explain the finding that approximately 10% of subjects taking T4 replacement therapy are distressed.57 Other investigators have found isolated elevations of tissue and serum IgG4 concentrations in Hashimoto's thyroiditis, but their significance remains uncertain.58 Intriguingly, Hashimoto's positive IgG4 patients are significantly younger than those IgG4-negative patients. Thus, Hashimoto's patients could potentially be divided into those that are IgG4 positive and IgG4 negative, and this classification might have important clinical implications.59
All these changes at a molecular level will be translated into hormonal fluctuations at the tissue level. The time to considerer a personalized (or “precise”) regimen of thyroid hormone replacement therapy in hypothyroid patients is at hand. Innovative formulations of thyroid hormones will be required to mimic a more perfect thyroid hormone replacement therapy than currently available.60 Precision medicine will provide information to tailor therapy to subcategories of disease or patients, defined by genomics, environmental or clinical data.
Small moleculesUnusual types of hypothyroidism are caused by mutations in the TSHR or the TSH molecule itself that confer TSH resistance or a diminished TSHR response, respectively. TSHR agonists could theoretically circumvent these defects. A potential additional advantage of treating hypothyroidism with TSHR agonists is that these compounds would stimulate the thyroid gland to produce both T4 and T3, which constitutes a clear benefit (mimicking physiological pathways) over the present therapy with levothyroxine alone.61
Pregnancy and thyroid dysfunctionOver the last few years, much attention has been paid to the unique features of the management of thyroid dysfunction in pregnant women.
Preventive measuresPublic health intervention: preventionPrecision medicine focuses largely on individual diagnosis and treatment, but public health interventions aimed at disease prevention might also be possible.62 The adverse consequences of hypothyroidism for mother and fetus have been well established.63,64 The concept of precision prevention may efficiently target specific subsets of pregnant women, with the highest cost/benefit ratio for TSH quantification and replacement therapy with levothyroxine.
DiagnosisPregnancy is a clinical situation in which the analytical accuracy in diagnosing thyroid dysfunction should be maximal. During pregnancy, the thyroid gland is under substantial additional pregnancy-related demands requiring physiological increases in thyroid hormone production. Furthermore, as opposed to other situations, serum TSH levels alone may be not adequate to completely assess thyroid function during gestation.
Normal TSH values in pregnancyPrecision medicine applied to thyroid function and dysfunction during pregnancy implies a clear-cut definition of trimester-specific reference ranges for thyroid hormones and TSH. The upper limit of the reference range for serum TSH has been intensely debated during the last few years. Again, in pregnancy, the “one size fits all” policy cannot be considered optimal medical practice. It is quite possible that the limits are not universal and will vary with patients’ ethnicity and geographic origin.65 Nevertheless, most of the current guidelines recommend using a TSH upper limit value of 2.5mU/l for preconception and first trimester, and 3.0mU/l for the second and third trimester.63,64,66 But, this recommendation is based on a limited number of studies. The lower limit of the serum TSH reference range during pregnancy is less worrying from the point of view of precision diagnostics, since subclinical hyperthyroidism seems to be accompanied by no special harmful effects to the mother and fetus.67 Recommendations of international societies for the lower limit are 0.1, 0.2 and 0.3mU/l for first, second, and third trimester, respectively.61,62,64 However, up to 20% of normal pregnant women display transiently low or undetectable serum TSH concentrations.68 It should be mentioned that in the process of defining population-based trimester-specific reference intervals, only women who are iodine sufficient and free of TPOAb should be included in the standardizing population.30
Isolated hypothyroxinemiaIsolated hypothyroxinemia occurs in 1–10% of pregnant women, depending on the definition, and is characterized by a decrease in the serum concentration of free T4 with normal TSH levels. It has been associated by some authors with offspring neurocognitive dysfunction.69 The diagnosis of this condition may be difficult due to pregnancy-related physiological changes in serum FT4, and because usual immunoassays in clinical laboratories do not reliably measure the concentration of FT4, especially in the second and third trimesters.70 In addition, there is no agreement among authors whether this condition should be diagnosed when the concentration of free T4 is below the 5th or the 10th percentile.71
Measurement of serum free T4 concentrations in the dialysate or ultrafiltration of serum samples using LC/MS has proven to be the most precise measurement of free T4 during pregnancy, which appears to decrease with advanced gestational age.63 This procedure is expensive and time-consuming, and is not readily available in most centers. A recommended alternative is to use trimester-specific immunoassay reference ranges, realizing that the results are often comparable or lower than in non pregnant controls due to pregnancy related causes.70 Given all these difficulties, a task of precision medicine will be to define with greater accuracy the biochemical diagnostic criteria, and the potential consequences, and treatment options of this form of thyroid dysfunction during pregnancy.
AutoimmunityAccurate quantification of TPOAb and TRAb is helpful for the differential diagnosis of autoimmune thyroid disease and other causes of thyroid dysfunction in pregnancy, as autoimmune activity decreases progressively during gestation.72 Women with positive TPOAb known before pregnancy may be at increased risk of developing hypothyroidism and obstetric complications during pregnancy.73 It is recommended that the serum TSH be measured every 4 weeks during the first half of gestation and at least once between week 26 and 32.63 Precision medicine should help us to decide how to screen pregnant women whose autoimmunity status is unknown. Current guidelines state that there is insufficient evidence to recommend antibody screening during the first trimester.64,65 There is no strong evidence to recommend treatment with LT4 in antibody positive euthyroid women, although one randomized trial showed benefit,74 this important question requires confirmatory studies. Some authors have used treatment with selenium to reduce levels of TPOAb in pregnancy and reduce the frequency of postpartum thyroid dysfunction,75 but its effectiveness in reducing obstetric complications has not been clearly demonstrated.
Postpartum thyroiditisPrecision medicine should offer new tools to avoid the risks of hypothyroidism during pregnancy and the development of postpartum thyroiditis. The most predictive factor for the development of postpartum thyroiditis is the TPOAb positivity.76 Even so, we still face a lack of specificity because at least 50% of seropositive women do not develop the condition. Our improved knowledge of the pathogenesis of thyroid diseases associated with pregnancy and postpartum will guide both treatments and preventive strategies in this setting.
Research needs, limitations and obstacles for precision thyroidologyThe genome and clinical connectionMany clinicians have been practicing personalized medicine for years now, using the best of the scientific evidence and knowledge. Precision medicine implies something different, though. It is a further step forward in accurately targeting the specific particularities of a disease in an individual patient. As we are pioneers in this effort, all the hypotheses, innovations, and recommendations in the field of precision medicine have to be tested in pilot studies. For the future implementation and extensive use of precision medicine in patients with thyroid dysfunction, we need a large amount of information, including clinically relevant data, selected hormonal and laboratory investigations, biological specimens, and behavioral and psychosocial data, and all have to be linked by electronic health records. However, specific genomic sequencing will potentially have a major impact, as it has the potential to split current nosologic classifications into different new subgroups. Proper analysis of this information will enable us to design rigorous interventional studies to address specific questions, including new opportunities for prevention and treatment of thyroid dysfunction.
In this regard, the connection between genomic and clinical data across cohorts is of significant importance. One of the potentially exciting results from connecting the health records of a very large number of individuals is the possibility of identifying patients with rare clinical or laboratory patterns that share similar signs and symptoms. In some cases, such approaches have been useful in defining novel syndromes, and allowing resources to flow toward targeted therapeutic development.77
The launching of a new era in medicineThis new era of precision medicine will become a scientific model that emphasizes engaged participants and open, responsible data sharing.1 To achieve the goals of precision medicine, a change in the paradigm of the regulatory framework of the health system is also needed. The National Institutes of Health of the United States proposed the creation of a national cohort of at least one million participants to support researchers and clinicians with a large database on genomic, environmental, and lifestyle information. This ambitious project will lead to new discoveries and treatments that will allow more precise management tailored to the specific characteristics of individual patients. Thus, the Precision Medicine Initiative,78 if properly developed, has the potential to identify genetic and environmental factors that might protect individuals from lethal diseases, but also this project could lead to potential new treatments and preventive measures.1,79 At this stage we can only imagine the potential role of artificial intelligence in this new era of precision medicine.
The future endocrinologist may deal with patients whose DNA has been already sequenced as part of their routine medical check-up. An endocrinologist might then take advantage of the identification of DNA markers that indicate the individual's susceptibility to thyroid disease, as well as treatments that are individually tailored.
Clinicians’ awareness about precision, and its possibilities in clinical practice should be advocated and supported by academic and health authorities.80 Medical schools have a duty to take the lead in educating future physicians and health workers about the new possibilities for treatment and prevention in the new era of precision medicine.
Potential hurdlesTo develop and implement precision medicine, multiple barriers will need to be overcome.81 The difficulties include the high costs of testing, the absence of reliable predictive biomarkers for most conditions, the lack of clear therapeutic alternatives (based on genetic differences) for many disorders, and the insufficient knowledge and expertise among most clinicians regarding genetics, risk prediction, and genetic counseling. In addition, the access to genetic information will raise complex ethical, legal, financial, and social issues.82 Ethical issues of genomic investigations and the protection of data will be of particular concern. Boundaries of patient privacy will need to be drawn. Gene manipulation is another issue that may arouse ethical concerns.
Some believe that precision medicine could create unrealistic expectations for patients and health providers. It is clear that the simple knowledge of a person's genome will not automatically enable the proper treatment to be developed. Another concern is the economic cost of these initiatives in developing countries, where other public health problems will take precedence.
It is noteworthy that in the particular case of thyroid dysfunction, we have been unable, as yet, to agree on the benefits of screening for hypothyroidism in the general population or in pregnancy. A precision medicine initiative in this area will require the collection and analysis of a great number of genetic, biological, psychosocial, and environmental variables in large cohorts of individuals. The question of how we could agree on the selection of suitable population samples and the implementation of research projects given the current economic constraints is not easy to address at present. Nevertheless, we believe that the potential benefits greatly outnumber the drawbacks. For instance, while it is true that the costs of implementing precision medicine can be very large, but there has been a dramatic decrease in the costs of DNA sequencing expenses in the last few years.
Concluding remarksPrecision medicine's promise is still much in the future, but it is not so distant as to be the realm of science fiction. The notion that personalized diagnostic and therapeutic approaches will encompass both a patient's genomic makeup and their environmental exposures (the “envirome”) should arouse great excitement and anticipation. As the personalized medicine initiative unfolds, clinicians who care for patients with thyroid dysfunction will pioneer the exploration of this new medical paradigm. We realize that challenges lie ahead; but the potential for clinical benefit is unequivocal and momentum is building. It is up to us to educate ourselves and our patients to embrace this new era in medical science.
Conflict of interestThe authors have nothing to disclose regarding the content of this article and no existing competing financial interests.