Debido a las limitaciones que tiene el ultrasonido endoscópico en la evaluación de la vascularidad de un tejido, el advenimiento de contrastes harmónicos que pueden administrarse en el momento del estudio y que consisten principalmente en microburbujas rellenas de aire, han permitido el desarrollo del ultra-sonido endoscópico (USE) con contraste harmónico, la cual es una herramienta prometedora para lograr diagnósticos por USE. Una ventaja de este método es la posibilidad de evaluar los patrones de realce en tiempo real. Una aplicación evidente es su posible utilidad en la diferenciación entre adenocarcinomas pancreáticos y pancreatitis crónica con seudotumores.
Because EUS has limitations in evaluating vascularity by US contrast when the color-Doppler or power-Doppler mode is used and because contrast harmonic imaging by intravenous infusion of Levovist®, an air-filled microbubble, has allowed the observation of the vasculature of the abdominal organs on transabdominal ultrasound (US) the idea of contrast-enhancement EUS (CE-EUS) is a very promising tool for diagnosis by EUS. An inherent advantage of CEEUS is the possibility to assess the contrast enhancement patterns in real time with a substantially higher temporal resolution than other imaging modalities. In pancreatic diseases, diagnosis between adenocarcinomas and nodular chronic pancreatitis are a big concern. It is possible that CE-EUS will be a useful tool for differential diagnosis and CE-EUS could provide a contribution to the differential diagnosis between a primary pancreatic carcinoma, chronic pancreatitis and a pancreatic metastasis.
Introduction
Among several technologies, endoscopic ultrasound (EUS) has been widely used to diagnose pancreatic, lymph nodes and gastrointestinal (GI) tumours, because EUS is superior to any other modality with respect to spatial resolution. However, EUS has limitations in evaluating vascularity by US contrast when the color-Doppler or power-Doppler mode is used. Contrast-enhanced power-Doppler US is accompanied by artefacts, e.g., blooming, so that the width of a blood vessel visualized by the power-Doppler mode is magnified and wider than that visualized by fundamental B-mode imaging. Contrast harmonic imaging by intravenous (IV) infusion of Levovist® (Schering AG, Berlin, Germany), an air-filled microbubble with an outer shell composed of 99.9% galactose and 0.1% palmitic acid, has allowed the observation of the vasculature of the abdominal organs on transabdominal ultrasound (US). If the US equipment receives harmonic components that are integer multiples of the fundamental frequency, then the harmonic content derived from microbubbles is higher than that from tissues. Contrast harmonic imaging detects signals from microbubbles and filters signals that originate from tissue by selectively detecting the harmonic components. This technology can detect signals from microbubbles in vessels with a very slow flow without Doppler-related artefacts and is used to characterize tumour vascularity in liver, pancreas, gallbladder, and the GI tract during transabdominal US. Until recently, there was no contrast harmonic imaging technique available for EUS examination, because the transducer for current echoendoscopes is of a limited frequency bandwidth and is too small to produce enough acoustic power for contrast harmonic imaging when using Levovist®. Second-generation US contrast agents, e.g., SonoVue® (Bracco Imaging, Milan, Italy), produce harmonic signals at lower acoustic powers and, therefore, are suitable for EUS imaging at low acoustic powers.
General considerations
US contrast agents (UCA), in conjunction with contrast specific imaging techniques, are increasingly accepted in clinical use for diagnostic imaging and post-interventional workup in several organs. To those not intimately involved in the field, the rapid advances in technology and techniques can be difficult to follow. In March of 2003, at the EUROSON
Congress in Copenhagen, it was agreed that it would be useful to produce a document providing a description of essential technical requirements, proposed investigator qualifications, suggested study procedures and steps, guidance on image interpretation, recommended and established clinical indications and safety considerations.1
The development of UCA, which perform as blood pool tracers, have overcome the limitations of conventional B-Mode and colour or power Doppler US and enable the display of parenchymal microvasculature.2 Dependent on the contrast agent and the US-mode, the dynamic lesion enhancement pattern is visualized during intermittent or continuous imaging. Enhancement patterns are described during subsequent vascular phases (e.g. arterial, portal venous and late phase for liver lesions), similar to contrast enhanced computer tomography (CECT) and/ or contrast enhanced magnetic resonance imaging (CEMRI). Contrast enhanced ultrasound (CEUS) and CECT or CEMRI are not equivalent as UCAs have different pharmacokinetics and are confined to the intravascular space, whereas the majority of currently approved contrast agents for CT and MRI are rapidly cleared from the blood pool into the extracellular space.
An inherent advantage of CEUS is the possibility to assess the contrast enhancement patterns in real time with a substantially higher temporal resolution than other imaging modalities, without the need to predefine scan time points or to perform bolus-tracking. Furthermore, administration can be repeated due to the excellent patient tolerance of UCAs. In addition to intravenous (IV) use, UCAs intracavity applications such as intravesical administration can be performed. UCA studies are subject to the same limitations as other types of ultrasound: as a general rule, if the baseline ultrasound is very suboptimal, CEUS may be disappointing.
Commercially available ultrasound contrast agents (UCA)
Four transpulmonary UCA are currently approved and marketed within European Countries (in Mexico until this moment there is not any available UCA):
1. Levovist® (air with a galactose and palmitic acid as a surfactant) (Schering, introduced in 1996). Main indications include heart, abdomen, vesico-ureteric reflux and transcranial.
2. Optison® (octafluoropropane [perflutren] with an albumin shell) (GE Healthcare, introduced in 1998). Sole indication is to date cardiac.
3. SonoVue® (sulfur hexafluoride with a phospholipid shell) (Bracco, introduced in 2001). Approved indications are cardiac (endocardial border delineation), macrovascular (cerebral and peripheral arteries, portal vein) and microvascular (characterisation of focal lesions in liver, pancreas and breast).
4. Luminity® (octafluoropropane perflutren with a lipid shell) (Bristol-Myers Squibb, introduced in 2006). Sole indication to date is cardiac.
The UCAs which are currently used in diagnostic US are characterized by a microbubble structure consisting of gas bubbles stabilized by a shell. UCA act as blood pool agents. They strongly increase the US backscatter and therefore are useful in the enhancement of echogenicity for the assessment of blood flow. While conventional ultrasound can detect high concentrations of microbubbles, in practice their assessment usually requires contrast specific imaging modes.
Contrast specific US modes are generally based on the cancellation and/or separation of linear US signals from tissue and utilization of the nonlinear response from microbubbles.
Non-linear response from microbubbles is based on two different mechanisms:
1. Non-linear response from microbubble oscillations at low acoustic pressure, chosen to minimize disruption of the microbubbles.
2. High energy broadband non-linear response arising from microbubble disruption. Non-linear harmonic US signals may arise also in tissues themselves due to a distortion of the sound wave during its propagation through the tissue. The extent of this harmonic response from tissue at a given frequency increases with the acoustic pressure, which is proportional to the mechanical index (MI).
Low solubility gas UCAs (e.g. SonoVue®, Optison®, Luminity®) are characterized by the combination of improved stability with favourable resonance behaviour at low acoustic pressure. This allows minimally disruptive contrast specific imaging at low MI and enables effective investigations over several minutes with the visualization of the dynamic enhancement pattern in real time. Low MI techniques furthermore lead to effective tissue signal suppression, as the non-linear response from the tissue is minimal when low acoustic pressures are used. US imaging with air filled microbubbles (e.g. Levovist®) at high pressure is dependent on microbubble disruption which is a significant limitation for real time imaging.
Pancreatic EUS and US constrast agents
Diagnosis between adenocarcinomas and nodular chronic pancreatitis is problematic. All methods of diagnosis are limited. Histology is the standard, but even biopsy can be difficult because cancers can produce a marked fibrotic reaction or necrosis, and give false results. For endoscopic retrograde cholangiopancreatography (ERCP), sensitivity and specificity are, respectively, 85% and 66% when there is a stenosis of the main pancreatic duct.3 Magnetic resonance cholangiopancreatography (MRCP) has a similar sensitivity and specificity for detecting pancreatic cancer or chronic pancreatitis as that of ERCP. Nevertheless, sensitivity is yet perfectible and MRCP gives a correct differentiation between malignant and benign lesions in 58% of cases.4,5 MRCP remains an expensive procedure, is time consuming, and is available only in a few centers.
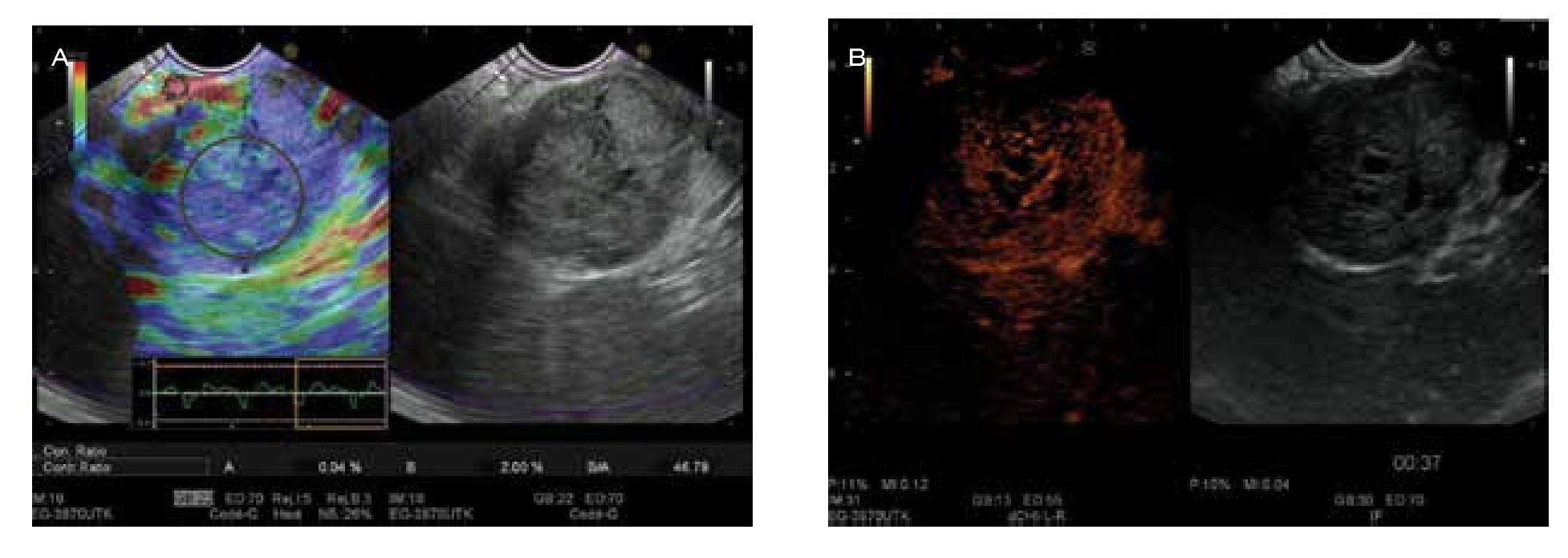
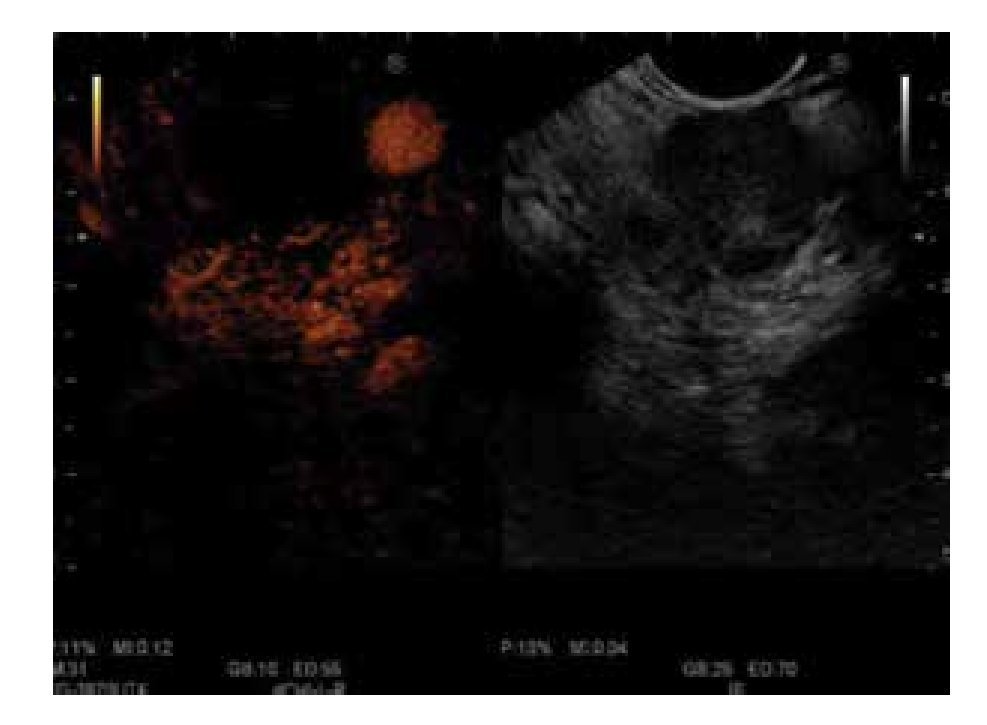
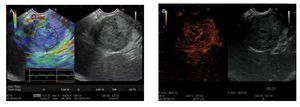
There are few studies about contrast-enhanced EUS (CE-EUS). In one of the first studies, Bhutani et al.6 evaluated the utility of SHU508 A (Levovist®) and concluded it could potentially improve the accuracy of EUS in the diagnosis of malignant vascular invasion, in detection of occult pancreatic neoplasms, and in the diagnosis of vascular thrombosis. Subsequently, Hirooka et al.7 studied the presence or absence of enhancement of different lesions with Albunex® in 37 patients. An enhancement of the lesion was observed in 100% of the patients with islet cell tumour (Figure 1), in 80% with intraductal papillary mucinous tumour (IPMT), in 75% with chronic pancreatitis, and no enhancement effect was observed in the patients with carcinoma (Figure 2). All patients underwent angiography, and comparison between images of CE-EUS and angiographic images showed similar results, except for three patients (two IPMT and one chronic pancreatitis) in whom angiograms were hypovascular, but enhancement effect was observed on EUS images. Becker et al.8 showed their experience in 23 patients with another contrast agent (FS 069 Optison®) and evaluated CEEUS as a method of differentiating inflammation and carcinoma based on perfusion characteristics. Markedly hyperperfused lesions were considered as inflammatory pseudotumours, whereas hypoperfused lesions compared to surrounding tissue were considered as carcinomas. Sensitivity for differentiation of pancreatic carcinoma vs. inflammatory changes was 94%, specificity 100%, positive predictive value (PPV) 100%, negative predictive value (NPV) 88%. These results are very similar to ours9 (sensitivity 90.9%, specificity 88.8%, PPV 88.2%, and NPV 91.4%). In our study,9 we also studied hyperechoic lesions (supposed not to be a pancreatic adenocarcinoma), and sensitivity was 88.8%, specificity 90.9%, PPV 91.4%, and NPV 88.2%. In future, CE-EUS could allow us to have a direct and reliable result (malignant or not) without waiting several days for histological findings. Perhaps it could also save time and money in limiting the use of expensive EUS needles. CE-EUS could be an interesting complement to EUS fine needle aspiration (FNA) concerning diagnosis accuracy. EUS FNA sensitivity and diagnosis accuracy are, respectively, 75%-92% and 79%-92%.8,10-16 First reason, EUS FNA is not realizable in 6%-9% of cases owing to vessel interpositions, duodenal stenosis, and tumoral hardness, particularly in chronic pancreatitis. Then, sensitivity of the EUS FNA is limited by uninterpretable material (bleeding or non-cellular samples) ranging from 9%-19%. Totally, the lack of sensitivity of EUS FNA ranges from 8%-25% of cases.8
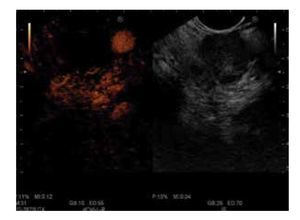
Figure 1. EUS aspects of endocrine tumour of the pancreas. A) Aspect with elastography mainly blue color and strain-ratio of 46.7 showing a hard mass; B) the enhancement of the micro-vascularization after SonoVue® injection makes the differential with adenocarcinoma.
Figure 2. No enhancement of the mass vascularization with peripherical hypervascularization after contrast injection: CEEUS image of pancreatic adenocarcinoma.
In our work,9 sensitivity and diagnostic accuracy of this technique were comparable to cytopathology results guided by EUS (sensitivity 90.9%, specificity 88.8%, PPV 88.2%, and NPV 91.4%). From a more general point of view, 97% of hypoechoic lesions were malignant tumours (30 adenocarcinoma, one endocrine tumour, one pancreatic lymphoma, one pancreatic metastasis from colonic cancer). Therefore, CE-EUS could appear like a reliable and complementary tool for EUS FNA in detection and classification of pancreatic lesions when EUS FNA is impossible or biopsy uninterpretable. CEEUS could improve accuracy and allow us to propose an appropriate treatment (surgery, follow-up, chemotherapy, etc.).
CE-EUS could allow us to differentiate malign tumour from pseudotumoral nodule. Chronic pancreatitis is also a limiting factor for diagnosis of pancreatic masses. Several works have attempted to establish EUS imaging criteria (without tissue sampling) for the discrimination of benign inflammatory pseudotumours and tumours. Despite the high resolution of EUS, it does not provide reliable differentiation of benign and malignant lesions of the pancreas.17 Fritscher-Ravens et al.19 found that sensitivity of EUS FNA in patients with a focal pancreatic lesion without chronic pancreatitis was 89%, while it was only 54% in patients with chronic pancreatitis. Nevertheless diagnosis of EUS FNA influenced clinical management in nearly half of patients.18 CEEUS could also play an important part in the case of lesions occurring within chronic pancreatitis. Indeed, in the study of Hocke et al.,3 adenocarcinoma developed on chronic pancreatitis was non enhanced after contrast injection. Conversely, pseudotumoral nodule (benign masses) (91%) in chronic pancreatitis was hypervascularized after SonoVue® injection. These results are different and better than CE-EUS. For Takeda et al.20 100% pseudotumoral pancreatitis had an isoenhanced pattern, and for Takeda et al.,20 it was difficult to differentiate adenocarcinomas from inflammatory pancreatic mass (50% were not well classified). In a recent meta-analysis by Gong et al.21 that includes 12 studies involving 1139 patients a pooled sensitivity of 94% and pooled specificity of 89% for differential diagnosis of pancreatic mass lesions was reported. The finding of a hypoenhanced lesion was a sensitive and accurate predictor of pancreatic adenocarcinoma.
CE-EUS could be useful in the case of negative results after EUS FNA. In early studies, NPV of EUSFNA was around 75%,13,14 but most recent studies found NPV between 26% and 44%.9-15 In the work from Oshikawa et al.,22 the rate of patients with negative results of the first biopsy, but with malignant tumour diagnosed a second time with a new puncture or with surgery, was 47%.
To conclude, negative predictive value of pancreatic EUS-FNA is 30%-33%. Theoretically, a new puncture is mandatory to be sure that it is normal tissue. We can also imagine that CE-EUS could avoid this second procedure. With regard to false-negative results of SonoVue®, we found three adenocarcinomas that presented hyperechoic aspect (enhancement contrast pattern). Two were poorly differentiated adenocarcinoma and the third was associated with IMPT. This suggests that poorly differentiated adenocarcinoma could have different vascularity of well-differentiated adenocarcinoma. These results were similar to studies with CE-EUS.23,24 Differences in histology, such as histological differentiation grade, amount of fibrosis, and obliteration of blood vessels in the tumour, may be associated with differences in enhancement behaviour.
Concerning CE -EUS and endocr ine tumours, there is only one case report using Levovist® that seemed to be a useful diagnostic method for precise localization of small insulinoma.25 In our study,9 87.5% (7/8) of endocrine tumours had a strong contrast-enhancement pattern, indicating hypervascular lesions. These results were similar to CE-EUS.19,23,24,26 These vascular images differed from those of almost all pancreatic ductal carcinomas. Thus, differentiation of enhancement pattern on CE-EUS between pancreatic adenocarcinomas and endocrine tumours is useful in the diagnosis of these lesions. In addition, "standard" EUS is already known to have a great value for localizing endocrine pancreatic tumours because of its excellent capacity to visualize small lesions and tumour vascularization at the same time.27,28 Therefore, we are authorized to think CE-EUS could increase sensitivity of diagnosis of pancreatic tumours.
Regarding IPMT, in our study,9 the only benign tumour was hyperechoic, whereas in malignant IPMT, one was hypoechoic and another was hyperechoic. In CE-EUS studies, malignancy could be associated with contrast enhancement. For Sofuni et al.,23 all (four patients) with IPMT showed hypervascularity of the nodules inside the tumours. For Nagase et al.,24 two of the five IPMT had solid components within the tumours and they were positive for enhancement effects. All five patients with IPMT underwent surgical resection and pathologic examination revealed malignancy in the two lesions with solid components and positive enhancement. For Itoh et al.,29 when the patients with carcinoma were compared with those with adenoma, the post-enhancement intensity was significantly higher in the carcinoma group. CE-EUS could be useful for the differential diagnosis of benign and malignant IPMT. The small number of patients with IMPT in each study did not allow conclusions. Finally, Yamashita et al.30 reported the diagnostic yield of CE-EUS for differentiation of mural nodule vs. mucous clots in patients with IPMN. They included 17 patients (12 nodules and five clots). CE-EUS depicted vascularity in all 12 cases and no vascularity in 4/5 mucous clots for an accuracy of 94%.
Metastatic lesions of the pancreas are rare, between 5%-10%,31 but an important cause of focal pancreatic lesions. There is only one description of one case of kidney metastasis analyzed in CE-EUS.32
Our work9 is the first in the literature that describes the enhancement pattern of pancreatic metastasis in CE-EUS. All metastasis except one (4/5; 80%) showed an echo enhancement pattern, probably proving their hypervascularization. The only pancreatic metastasis non-enhanced was from colonic cancer. CE-EUS could provide a contribution to the differential diagnosis between a primary pancreatic carcinoma and a pancreatic metastasis, and therefore can have a decisive influence on the selection of appropriate therapeutic strateg ies (chemotherapy rather than surgery, for example). However, histology remains the standard in the differential diagnosis of pancreatic tumours.
Contrast-enhanced endoscopic ultrasound in discrimination between benign and malignant mediastinal and abdominal lymph nodes
Enlarged mediastinal or abdominal lymph nodes without a causing disease is nowadays a clinical issue because of the rapid development of imaging techniques with an increasingly higher resolution of intrathoracic or intraabdominal structures. Current computed tomography scanners reliably recognize lymph nodes of 5-10 mm in size, but do not allow distinguishing between malignant and benign lymph nodes in the majority of cases. Endoscopic ultrasound has an even higher local resolution, but again, given a distinct visible lymph node, the investigator is frequently not able to assign this node to a malignancy whether or not a malignant tumour is already known. There are several ultrasound features such as size, round shape, hypoechoic appearance, missing "hilus sign" and clear borders which cumulatively make a malignant lymph node most likely, but on the one hand the specificity for these signs is still below 90% and, on the other, the majority of malignant lymph nodes do not exhibit all these signs simultaneously. The EUS-guided fine-needle aspiration (FNA) represents the current "gold-standard" and has replaced more invasive procedures like mediastinoscopy at least for those groups of lymph nodes, which are in reach of the endoscopic ultrasound. In the hands of trained investigators, EUS-FNA reaches a sensitivity, specificity and diagnostic accuracy of over 90%. Hocke et al33 had investigated a total of 122 patients with enlarged mediastinal and/or paraaortic lymph nodes diagnosed by CT scan were included in the study. EUS-guided fine needle aspiration was performed and cytologic specimens were diagnosed as representing a malignant or benign process in case of Papanicolaou IV and V, or Papanicolaou I and II, respectively. Based on cytology results, the investigated lymph nodes were classified as neoplastic (n=48) or non-neoplastic lymph nodes. Using the B-mode criteria the preliminary diagnosis was confirmed in 64 out of 74 benign lymph nodes (specificity 86%). Regarding malignant lymph nodes 33 of 48 were confirmed (sensitivity 68%). Using the advanced contrast-enhanced EUS criteria the diagnosis was confirmed in 68 of 74 benign lymph nodes (specificity 91%). However, in case of malignant lymph nodes the number of correct diagnoses dropped to 29 of 48 lymph nodes (sensitivity 60%). The contrast-enhanced EUS criteria to identify benign lymph nodes and node enlargement in malignant lymphoma do not differ. If those ten patients with malignant lymphoma are excluded, the sensitivity of the contrast enhanced EUS for malignant lymph nodes rises to 73%. Contrast-enhanced EUS improves the specificity in diagnosing benign lymph nodes as compared to B mode EUS. It does not improve the correct identification of malignant lymph nodes and cannot replace EUS-guided fine-needle aspiration.34
In general, we can say that with CE-EUS malignant nodes has peripherical enhancement, inflammatory nodes has centrifugal enhancement and lesions as lymphoma has intense homogeneous enhancement.35
Conclusion
CE-EUS could provide a contribution to the differential diagnosis between a primary pancreatic carcinoma, chronic pancreatitis and a pancreatic metastasis, and therefore can have a decisive influence on the selection of appropriate therapeutic strategies (follow-up, chemotherapy or surgery, for example). However, histology remains the standard in the differential diagnosis of pancreatic tumours. Regarding lymph nodes, CE-EUS cannot replace EUS-guided fine-needle aspiration.
Conflict of interest
The authors declare no conflict of interest.
Funding
None.
Corresponding author:
Félix I. Téllez-Ávila M.D. M.Sc. Ph.D.
Endoscopy Department,
Instituto Nacional de Ciencias Médicas y Nutrición "Salvador Zubirán".
Vasco de Quiroga 15, Col. Sección XVI, Tlalpan, Mexico City, Mexico.
E-mail: felixtelleza@gmail.com