The main aim of this study was to assess changes in the epidemiology and clinical presentation of Acinetobacter baumannii over a 10-year period, as well as risk factors of mortality in infected patients.
MethodProspective, multicentre, hospital-based cohort studies including critically ill patients with A. baumannii isolated from any clinical sample were included. These were divided into a first period (“2000 study”) (one month), and a second period (“2010 study”) (two months). Molecular typing was performed by REP-PCR, PFGE and MSLT. The primary endpoint was 30-day mortality.
ResultsIn 2000 and 2010, 103 and 108 patients were included, and the incidence of A. baumannii colonization/infection in the ICU decreased in 2010 (1.23 vs. 4.35 cases/1000 patient-days; p<0.0001). No differences were found in the colonization rates (44.3 vs. 38.6%) or infected patients (55.7 vs. 61.4%) in both periods. Overall, 30-day mortality was similar in both periods (29.1 vs. 27.8%). The rate of pneumonia increased from 46.2 in 2000 to 64.8% in 2010 (p<0.001). Performing MSLT, 18 different sequence types (ST) were identified (18 in 2000, 8 in 2010), but ST2 and ST79 were the predominant clones. ST2 isolates in the ICU increased from 53.4% in the year 2000 to 73.8% in 2010 (p=0.002). In patients with A. baumannii infection, the multivariate analysis identified appropriate antimicrobial therapy and ST79 clonal group as protective factors for mortality.
ConclusionsAt 10 years of the first analysis, some variations have been observed in the epidemiology of A. baumannii in the ICU, with no changes in mortality. Epidemic ST79 clone seems to be associated with a better prognosis and adequate treatment is crucial in terms of survival.
El principal objetivo fue evaluar los cambios en la epidemiología a lo largo de un periodo de 10años, así como la presentación clínica y los factores predictores de mortalidad en los pacientes críticos infectados por Acinetobacter baumannii.
MétodoEstudio de cohortes prospectivo y multicéntrico en el que se incluyeron pacientes críticos con A. baumannii aislado de cualquier muestra clínica. Se consideró un primer período («estudio de 2000») (un mes) y un segundo («estudio de 2010») (2 meses). La tipificación molecular se realizó mediante REP-PCR, PFGE y MSLT. La variable resultado primaria fue la mortalidad a los 30días.
ResultadosEn 2000 y 2010 se incluyeron 103 y 108 pacientes, respectivamente, y la incidencia de colonización/infección por A. baumannii en la UCI disminuyó en 2010 respecto al 2000 (1,23 vs. 4,35 casos/1.000 pacientes-días; p<0,0001). No se encontraron diferencias en la tasa de colonización (44,3 vs. 38,6%) o infección (55,7 vs. 61,4%) en ambos periodos. En general, la mortalidad a los30 días fue similar en ambos periodos (29,1 vs. 27,8%). La tasa de neumonía aumentó desde el 46,2% en 2000 al 64,8% en 2010 (p<0,001). Mediante MSLT, se identificaron 18 tipos de secuencias diferentes (ST) (18 en 2000, 8 en 2010), pero ST2 y ST79 fueron los clones predominantes. La identificación de ST2 aumentó en la UCI desde el 53,4% en 2000 al 73,8% en 2010 (p=0,002). En los pacientes infectados, el tratamiento antimicrobiano apropiado y el grupo clonal ST79 fueron factores protectores de mortalidad en el análisis multivariante.
ConclusionesA los 10años del primer análisis se han observado algunos cambios en la epidemiología de A. baumannii en la UCI, sin cambios en la mortalidad. El clon ST79 epidémico parece estar asociado con un mejor pronóstico, y el tratamiento adecuado es crucial en términos de supervivencia.
Acinetobacter baumannii constitutes an increasing problem worldwide. Outbreaks of nosocomial infections and endemic situations caused by multidrug-resistant A. baumannii have reached the proportions of a national health problem causing great social alarm in several countries. This situation is especially serious and troublesome in the Intensive Care Units (ICU). In fact, A. baumannii was the fifth most common pathogen in a prevalence study of infections in ICU conducted in 75 countries of the five continents.1A. baumannii has also constituted a serious problem in Spanish ICU during the previous decade.2
The most frequent clinical manifestations of A. baumannii infection in the critical care setting are ventilator-associated pneumonia and bloodstream infection. Although A. baumannii has been considered as a pathogen with limited virulence, invasive infections, especially those caused by multi-drug resistant strains, are associated with increased morbidity and mortality in predisposed patients.3
In a study performed in 2000 in Spain, 40% of A. baumannii isolates were resistant to carbapenems;4 at that time, these antimicrobials were the treatment of choice. However, the rate of the resistance to carbapenems has increased dramatically in the last decade, especially in the critical care setting.5 Frequently, colistin constitutes the only therapeutic option because of the high rate of resistance to carbapenems and the other previous therapeutic alternatives (i.e. sulbactam).6
The genetic analysis of A. baumannii isolates provides valuable data regarding their epidemic distribution. Such studies usually reveal the clonal nature of A. baumannii isolates, not only within single institutions, but also on a global basis. Diverse studies have assessed the genotypic characterization of A. baumannii isolated in the critical care setting.7,8 Nevertheless, the impact of A. baumannii strain type on the outcome in patients with A. baumannii infections has not been extensively analyzed.8,9
We have previously investigated the clinical and molecular epidemiology of A. baumannii in the whole hospital setting in Spain.10,11 However, since A. baumannii transmission is particularly important in the ICU setting and the clinical consequences of this organism in ICU patients are usually more devastating than in patients in general wards, we decided to perform a sub-analysis of ICU patients. Our objectives were: (1) to reassess the epidemiology, microbiological and clinical features of A. baumannii isolated from patients admitted to the ICU; (2) to compare the current information with the situation ten years before and (3) to determine the factors associated with 30-day mortality of A. baumannii infections in critically ill patients.
MethodsStudy design, sites and participantsTwo prospective multicenter, hospital-based cohort studies were performed, using the same methodology, Twenty-seven Spanish acute care hospitals participated in the first study (“2000 cohort”) that was carried out from November 1 to November 30, 2000 (patients from a specialized center for paraplegic patients were excluded from this analysis).10 The second (“2010 cohort”) study was undertaken in 38 Spanish acute care hospitals between February 1 and March 31, 2010.11
Patients from whom A. baumannii was isolated from any clinical sample were prospectively included in the cohorts, as detected daily by reviewing the microbiology reports in the participating centers. Active surveillance samples (such as samples performed to detect colonization for infection control purposes) were not considered. Patients were excluded if they were colonized or infected by A. baumannii during the previous year. All patients were followed for 30 days after isolation of the A. baumannii.
The study was approved by the Ethics Committee of the Hospital Universitario Virgen Macarena. The need to obtain informed consent was waived due to the observational nature of the study.
Variables and definitionsThe following data were collected: age, gender, type of acquisition, ward of admission, chronic underlying diseases and the Charlson Comorbidity Index,12 severity of the underlying condition according to the McCabe classification,13 invasive procedures, antibiotic use in the previous 2 months, community or nosocomial acquisition, infection or colonization and type of infection, according to CDC criteria,14 and mechanical ventilation the day of culture that yielded A. baumannii. Isolation of A. baumannii in a clinical sample together with the presence of clinical signs of infection was not sufficient to regard the patient as infected by A. baumannii; other sources of infection had to have been ruled out. Ventilator-associated pneumonia diagnosis required radiographic image of a new and persistent pulmonary infiltrate and at least two of the following criteria: temperature >38°C or <35.5°C, leukocytosis above 12,000cells/mm3 or leukopenia below 4000cells/mm3, and purulent bronchial secretions. Microbiological diagnosis was obtained with quantitative culture of lower respiratory tract samples (tracheal aspirate or bronchoalveolar lavage).15 When it was not possible to identify whether the status of the patient was infected or colonized, the patient was considered to be only colonized.
Severity of systemic inflammatory response was classified as sepsis, severe sepsis or septic shock following current definitions.16 Community or nosocomial acquisition was classified according to CDC definitions;14 community acquisition was considered as healthcare-associated if any of the following was demonstrated: hospital admission during the previous year; and/or attention at a day hospital, specialized care home, or dialysis in the previous 3 months.
The physician in charge of the patient chose antimicrobial treatment. Empirical therapy was considered appropriate if at least one drug active in vitro against the isolated strain was administered during the first 24h. Therapy with two or more active drugs was considered as combination therapy which included a non-active in vitro carbapenem were considered combination therapy if the carbapenem minimum inhibitory concentration (MIC) was lower than 32mg/L.17
Patients were followed up until discharge or death, or until 30 days after the sample had been obtained if the patient was still hospitalized.
Microbiological studiesAll isolates presumptively identified as Acinetobacter spp. at each center by conventional methods were sent to the reference laboratories for definitive identification and genotyping as explained in detail elsewhere.11 Only patients whose isolates were definitively identified as A. baumannii were included. At the reference laboratory, microdilution susceptibility testing was performed, according to CLSI recommendations.18 For sulbactam, tigecycline and rifampin, isolates with an MIC of ≤8mg/L, 1 and 4mg/L, respectively, were considered as susceptible.19 Clonal relationships between the first isolates obtained from each patient were determined by repetitive extragenic palindromic PCR [REP-PCR].20,21
Representative strains of each REP-PCR profile at each hospital were also studied by pulsed-field gel electrophoresis (PFGE),6 and by multilocus sequence typing (MLST), following the protocol developed by the Pasteur Institute (www.pasteur.fr) as previously explained in detail.10 Isolates of the same REP-PCR and PFGE types were assigned the sequence type (ST) found for the representative isolate studied by MSLT. For statistical purposes, sporadic isolates were defined as those causing 3 cases or less.
Statistical analysisDiscrete variables were expressed as counts (percentages) and continuous variables as means±standard deviation. The χ2 test or Fisher's exact test was used for categorical variables, and the Mann–Whitney U test or Kruskal–Wallis test was used for continuous variables. To identify independent variables associated with 30-day mortality, we performed a multivariate analysis considered clones as independent categorical variable: the clone ST2 was taken as the reference category, as second category the clone ST79, and third, the remaining clones. To avoid spurious associations, variables entered into the regression models were those with a relationship in univariate analysis (p<0.05), with a plausible relationship with the dependent variable, or that were clinically significant. Results are presented as odds ratios (ORs) and 95% confidence intervals (CIs). Potential explanatory variables were checked for colinearity before inclusion in the regression models using the tolerance and variance inflation factor. The threshold for statistical significance was defined as p<0.05. Data analysis was performed using SPSS for Windows 19.0 (SPSS, Inc., Chicago, IL).
ResultsOverall, 246 patients with colonization or infection due to A. baumannii were detected in 30 of the 38 hospitals included in the 2010 cohort; in the 2000 cohort, 183 patients were detected in 25 of 27 acute care hospitals. In these two cohorts, A. baumannii was isolated in 108 and 103 patients in the ICU, respectively. In these 108 critically ill patients, A. baumannii was considered as etiologic agent of an infection in 71 patients and in 37 cases it was considered as a mere colonizer. The incidence density rate decreased significantly in 2010 in comparison to 2000 in the ICUs (1.23 vs. 4.35 cases/1000 patient-days; p<0.0001).
Predisposing factors, clinical features and outcome in both cohorts (ICU vs. non-critically ill patients)In both cohorts, A. baumannii was isolated in 211 patients at the ICU and in 218 in general wards. Critically ill patients with A. baumannii were younger (59 vs. 67 years) and with less underlying comorbidities than patients in other hospital wards (Charlson 0 vs. 1 points respectively). Pneumonia was the most frequent source of A. baumannii infection in ICU patients (55.9 vs. 17.1% in ICU and non-ICU patients respectively) whereas skin and soft-tissue infection (6.6 vs. 38.6% in ICU and non-ICU patients) and urinary tract (2.2 vs. 18.8% in ICU and non-ICU patients) were the predominant foci in non-ICU patients. Regarding clonality, ST2 clone was more frequently isolated in ICU patients than in non-ICU patients (63.5 vs. 52.7%; p=0.018). Mortality in ICU patients was 28.4% and 13.3% in non-ICU patients (p<0.001).
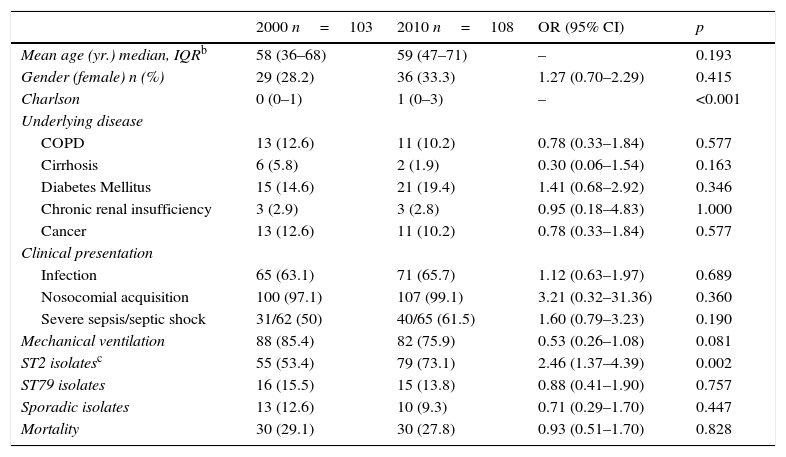
Predisposing factors, clinical features and outcome in critically ill patients with A. baumannii isolation (comparison 2010 vs. 2000)Demographic and clinical characteristics of the patients with A. baumannii isolation comparing 2010 with 2000 cohort are depicted in Table 1. Presence of underlying comorbidities was similar in both study periods. In a similar proportion of cases, A. baumannii was considered as mere colonizer. Nevertheless, rate of pneumonia as source of A. baumannii infections rose in 2010 in comparison with the 2000 cohort. Mortality was not statistically different in both study periods (29.1 vs. 27.8%; p=0.83).
Baseline features among patients with A. baumannii isolation in the ICUa in 2000 and 2010 cohorts.
| 2000 n=103 | 2010 n=108 | OR (95% CI) | p | |
|---|---|---|---|---|
| Mean age (yr.) median, IQRb | 58 (36–68) | 59 (47–71) | – | 0.193 |
| Gender (female) n (%) | 29 (28.2) | 36 (33.3) | 1.27 (0.70–2.29) | 0.415 |
| Charlson | 0 (0–1) | 1 (0–3) | – | <0.001 |
| Underlying disease | ||||
| COPD | 13 (12.6) | 11 (10.2) | 0.78 (0.33–1.84) | 0.577 |
| Cirrhosis | 6 (5.8) | 2 (1.9) | 0.30 (0.06–1.54) | 0.163 |
| Diabetes Mellitus | 15 (14.6) | 21 (19.4) | 1.41 (0.68–2.92) | 0.346 |
| Chronic renal insufficiency | 3 (2.9) | 3 (2.8) | 0.95 (0.18–4.83) | 1.000 |
| Cancer | 13 (12.6) | 11 (10.2) | 0.78 (0.33–1.84) | 0.577 |
| Clinical presentation | ||||
| Infection | 65 (63.1) | 71 (65.7) | 1.12 (0.63–1.97) | 0.689 |
| Nosocomial acquisition | 100 (97.1) | 107 (99.1) | 3.21 (0.32–31.36) | 0.360 |
| Severe sepsis/septic shock | 31/62 (50) | 40/65 (61.5) | 1.60 (0.79–3.23) | 0.190 |
| Mechanical ventilation | 88 (85.4) | 82 (75.9) | 0.53 (0.26–1.08) | 0.081 |
| ST2 isolatesc | 55 (53.4) | 79 (73.1) | 2.46 (1.37–4.39) | 0.002 |
| ST79 isolates | 16 (15.5) | 15 (13.8) | 0.88 (0.41–1.90) | 0.757 |
| Sporadic isolates | 13 (12.6) | 10 (9.3) | 0.71 (0.29–1.70) | 0.447 |
| Mortality | 30 (29.1) | 30 (27.8) | 0.93 (0.51–1.70) | 0.828 |
Using MSLT to determine clonality of the isolates in the ICU, 18 different STs (18 in 2000, 8 in 2010) were identified. Predominant STs were: ST2 (134 isolates [63.8%] from 16 ICU), ST79 (31 isolates [14.8%] from 8 hospitals), ST181 (7 isolates [6.9%] from 1 hospitals), and ST179 (3 isolates [4.2%] from 2 hospitals). The STs clones 2, 79,169, 181, 187, 264, 270 and 271 were found in both cohorts. ST179 was found in 2 hospitals in 2000 and in none in 2010.
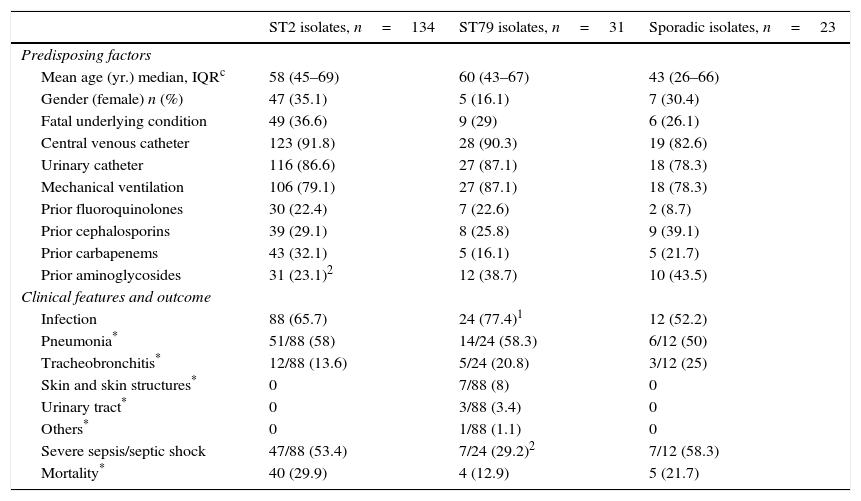
The proportion of ST2 isolates in the ICU increased from 53.4% in 2000 to 73.1% in 2010 (p=0.002). There was no apparent regional clustering. ST79 caused a similar percentage of cases in both study periods (15.5 vs. 13.8%; p=0.75). It was isolated at 4 ICU in the same region in 2000 and was not detected in any of them in 2010, when it was also found in other four centers located at a considerable distance from the others. Predisposing factors and clinical features of patients at the ICU with A. baumannii isolates belonging to ST2, ST79 or sporadic clones are shown in Table 2. ST2, ST79 and sporadic clones produced infection or colonization in a similar proportion of cases.
| ST2 isolates, n=134 | ST79 isolates, n=31 | Sporadic isolates, n=23 | |
|---|---|---|---|
| Predisposing factors | |||
| Mean age (yr.) median, IQRc | 58 (45–69) | 60 (43–67) | 43 (26–66) |
| Gender (female) n (%) | 47 (35.1) | 5 (16.1) | 7 (30.4) |
| Fatal underlying condition | 49 (36.6) | 9 (29) | 6 (26.1) |
| Central venous catheter | 123 (91.8) | 28 (90.3) | 19 (82.6) |
| Urinary catheter | 116 (86.6) | 27 (87.1) | 18 (78.3) |
| Mechanical ventilation | 106 (79.1) | 27 (87.1) | 18 (78.3) |
| Prior fluoroquinolones | 30 (22.4) | 7 (22.6) | 2 (8.7) |
| Prior cephalosporins | 39 (29.1) | 8 (25.8) | 9 (39.1) |
| Prior carbapenems | 43 (32.1) | 5 (16.1) | 5 (21.7) |
| Prior aminoglycosides | 31 (23.1)2 | 12 (38.7) | 10 (43.5) |
| Clinical features and outcome | |||
| Infection | 88 (65.7) | 24 (77.4)1 | 12 (52.2) |
| Pneumonia* | 51/88 (58) | 14/24 (58.3) | 6/12 (50) |
| Tracheobronchitis* | 12/88 (13.6) | 5/24 (20.8) | 3/12 (25) |
| Skin and skin structures* | 0 | 7/88 (8) | 0 |
| Urinary tract* | 0 | 3/88 (3.4) | 0 |
| Others* | 0 | 1/88 (1.1) | 0 |
| Severe sepsis/septic shock | 47/88 (53.4) | 7/24 (29.2)2 | 7/12 (58.3) |
| Mortality* | 40 (29.9) | 4 (12.9) | 5 (21.7) |
p values: 1 0.05–0.09; 2 0.04–0.01; all others, ≥0.05.
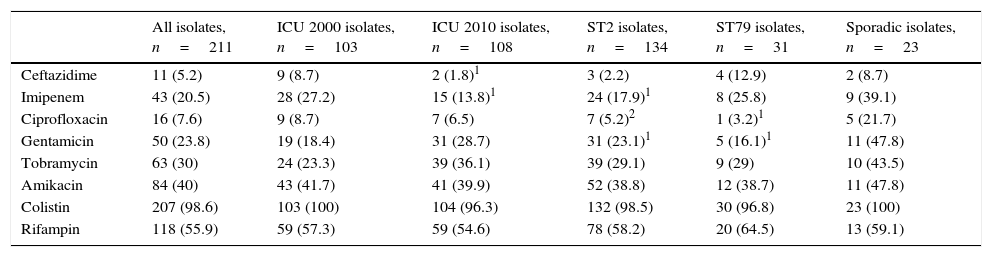
The susceptibility data of all isolates in the ICU by study period, and of ST2, ST79 and sporadic isolates are shown in Table 3. Overall, A. baumannii isolates were less frequently susceptible to ceftazidime and imipenem in 2010 compared to 2000; ST2 and ST79 were less frequently susceptible to ciprofloxacin and gentamicin, compared to sporadic isolates, with ST2 also being less susceptible to imipenem (17.9 vs. 39.1% p=0.02). ST79 isolates were less frequently susceptible to imipenem in 2010 compared to 2000 (6.7 vs. 43.8%, p=0.037). Colistin remains as the most active agent with independence of the clone. Rate of susceptibility to imipenem was not statistically different in ST2 and in ST79 clones (17.9 vs. 25.8%; p=0.316).
Susceptibility data for A. baumannii isolates from centers taking part in both 2000 and 2010 ICU cases. Data are expressed as percentages of susceptible isolates. Statistical comparisons were performed for 2010 vs. 2000 isolates, and for ST2 or ST79 vs. sporadic isolates.
| All isolates, n=211 | ICU 2000 isolates, n=103 | ICU 2010 isolates, n=108 | ST2 isolates, n=134 | ST79 isolates, n=31 | Sporadic isolates, n=23 | |
|---|---|---|---|---|---|---|
| Ceftazidime | 11 (5.2) | 9 (8.7) | 2 (1.8)1 | 3 (2.2) | 4 (12.9) | 2 (8.7) |
| Imipenem | 43 (20.5) | 28 (27.2) | 15 (13.8)1 | 24 (17.9)1 | 8 (25.8) | 9 (39.1) |
| Ciprofloxacin | 16 (7.6) | 9 (8.7) | 7 (6.5) | 7 (5.2)2 | 1 (3.2)1 | 5 (21.7) |
| Gentamicin | 50 (23.8) | 19 (18.4) | 31 (28.7) | 31 (23.1)1 | 5 (16.1)1 | 11 (47.8) |
| Tobramycin | 63 (30) | 24 (23.3) | 39 (36.1) | 39 (29.1) | 9 (29) | 10 (43.5) |
| Amikacin | 84 (40) | 43 (41.7) | 41 (39.9) | 52 (38.8) | 12 (38.7) | 11 (47.8) |
| Colistin | 207 (98.6) | 103 (100) | 104 (96.3) | 132 (98.5) | 30 (96.8) | 23 (100) |
| Rifampin | 118 (55.9) | 59 (57.3) | 59 (54.6) | 78 (58.2) | 20 (64.5) | 13 (59.1) |
ST: sequence type.
p values: 1 0.04–0.01; 2 0.009–0.001. All others, ≥0.05.
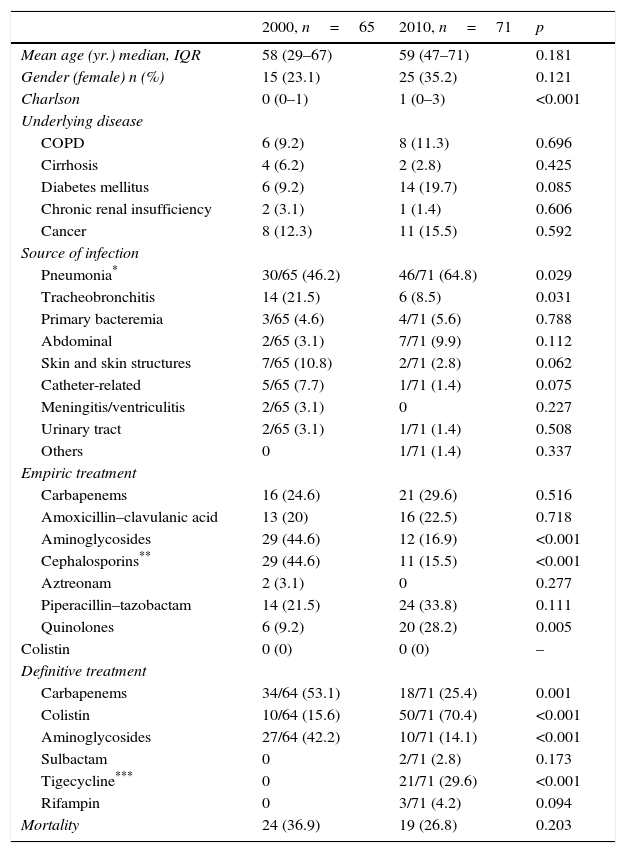
To determine clinical and microbiological factors associated with 30-day mortality, we analyzed all patients (n=136) with the diagnosis of infection in both cohorts. Therefore, we excluded 38 patients in 2000 cohort and 37 patients in the 2010 cohort with the diagnosis of A. baumannii colonization. Mortality of infected patients was 31.6% (43 patients). As shown in Table 4, mortality rate in infected patients was not statistically different in both periods (36.9% vs. 26.8%).
Source of infections, antimicrobial therapy, and outcome in patients with A. baumannii infection.
| 2000, n=65 | 2010, n=71 | p | |
|---|---|---|---|
| Mean age (yr.) median, IQR | 58 (29–67) | 59 (47–71) | 0.181 |
| Gender (female) n (%) | 15 (23.1) | 25 (35.2) | 0.121 |
| Charlson | 0 (0–1) | 1 (0–3) | <0.001 |
| Underlying disease | |||
| COPD | 6 (9.2) | 8 (11.3) | 0.696 |
| Cirrhosis | 4 (6.2) | 2 (2.8) | 0.425 |
| Diabetes mellitus | 6 (9.2) | 14 (19.7) | 0.085 |
| Chronic renal insufficiency | 2 (3.1) | 1 (1.4) | 0.606 |
| Cancer | 8 (12.3) | 11 (15.5) | 0.592 |
| Source of infection | |||
| Pneumonia* | 30/65 (46.2) | 46/71 (64.8) | 0.029 |
| Tracheobronchitis | 14 (21.5) | 6 (8.5) | 0.031 |
| Primary bacteremia | 3/65 (4.6) | 4/71 (5.6) | 0.788 |
| Abdominal | 2/65 (3.1) | 7/71 (9.9) | 0.112 |
| Skin and skin structures | 7/65 (10.8) | 2/71 (2.8) | 0.062 |
| Catheter-related | 5/65 (7.7) | 1/71 (1.4) | 0.075 |
| Meningitis/ventriculitis | 2/65 (3.1) | 0 | 0.227 |
| Urinary tract | 2/65 (3.1) | 1/71 (1.4) | 0.508 |
| Others | 0 | 1/71 (1.4) | 0.337 |
| Empiric treatment | |||
| Carbapenems | 16 (24.6) | 21 (29.6) | 0.516 |
| Amoxicillin–clavulanic acid | 13 (20) | 16 (22.5) | 0.718 |
| Aminoglycosides | 29 (44.6) | 12 (16.9) | <0.001 |
| Cephalosporins** | 29 (44.6) | 11 (15.5) | <0.001 |
| Aztreonam | 2 (3.1) | 0 | 0.277 |
| Piperacillin–tazobactam | 14 (21.5) | 24 (33.8) | 0.111 |
| Quinolones | 6 (9.2) | 20 (28.2) | 0.005 |
| Colistin | 0 (0) | 0 (0) | – |
| Definitive treatment | |||
| Carbapenems | 34/64 (53.1) | 18/71 (25.4) | 0.001 |
| Colistin | 10/64 (15.6) | 50/71 (70.4) | <0.001 |
| Aminoglycosides | 27/64 (42.2) | 10/71 (14.1) | <0.001 |
| Sulbactam | 0 | 2/71 (2.8) | 0.173 |
| Tigecycline*** | 0 | 21/71 (29.6) | <0.001 |
| Rifampin | 0 | 3/71 (4.2) | 0.094 |
| Mortality | 24 (36.9) | 19 (26.8) | 0.203 |
Types of infections, treatments, and crude mortality in patients with A. baumannii infection are depicted in Table 4. The episodes of respiratory infections have significantly increased in 2010 in comparison with the 2000 cohort. In contrast, other types of infection such as skin and soft tissue infection or ventriculitis/meningitis have markedly decreased. The use of colistin in the definitive therapy is more frequent in the 2010 cohort whereas the use of carbapenem or aminoglycosides has significantly decreased.
Twenty-one patients were treated with tigecycline in 2010 (loading dose of 100mg, followed by 50mg every 12h was used in all patients). The most frequent indication for tigecycline use was pneumonia (n=15). Mortality of patients treated with tigecycline was 28.6% (6/21) and 32.2% in the rest of infected patients (p=0.774). Nineteen patients treated with tigecycline received at least another active antimicrobial; being colistin the most frequently used agent (in 16 patients; 76.2%).
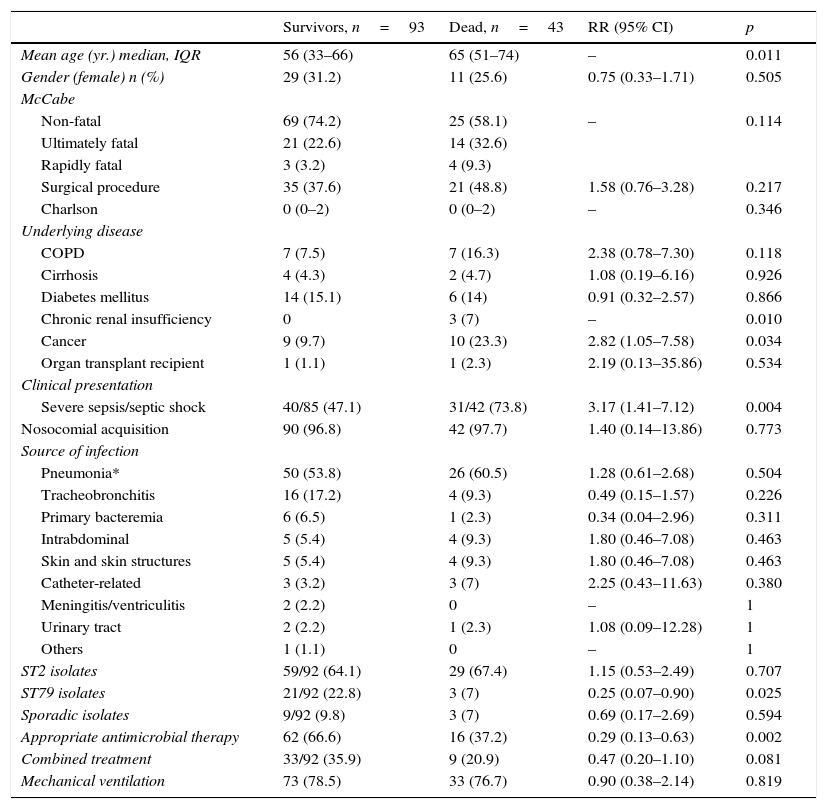
Table 5 shows the comparison between survivors and non-survivors in infected patients. In the multivariate analysis, only appropriate antimicrobial therapy (OR 0.11; 95% CI 0.02–0.51; p=0.004) and clone ST79 were protective factors of mortality (OR 0.07; 95% CI 0.008–0.76; p=0.028). Appropriate antimicrobial therapy was more often administered in infections caused by the ST2 clone than in infection caused by the genotype ST79 although this difference did not reach statistical significance (68.8 vs. 45.9%; p=0.060). Patients who survived received more frequently combination therapy although this difference was not statistically different and this factor was not included in the final multivariate model.
Bivariate analysis of hospital mortality in the total cohort of infected critically ill patients.
| Survivors, n=93 | Dead, n=43 | RR (95% CI) | p | |
|---|---|---|---|---|
| Mean age (yr.) median, IQR | 56 (33–66) | 65 (51–74) | – | 0.011 |
| Gender (female) n (%) | 29 (31.2) | 11 (25.6) | 0.75 (0.33–1.71) | 0.505 |
| McCabe | ||||
| Non-fatal | 69 (74.2) | 25 (58.1) | – | 0.114 |
| Ultimately fatal | 21 (22.6) | 14 (32.6) | ||
| Rapidly fatal | 3 (3.2) | 4 (9.3) | ||
| Surgical procedure | 35 (37.6) | 21 (48.8) | 1.58 (0.76–3.28) | 0.217 |
| Charlson | 0 (0–2) | 0 (0–2) | – | 0.346 |
| Underlying disease | ||||
| COPD | 7 (7.5) | 7 (16.3) | 2.38 (0.78–7.30) | 0.118 |
| Cirrhosis | 4 (4.3) | 2 (4.7) | 1.08 (0.19–6.16) | 0.926 |
| Diabetes mellitus | 14 (15.1) | 6 (14) | 0.91 (0.32–2.57) | 0.866 |
| Chronic renal insufficiency | 0 | 3 (7) | – | 0.010 |
| Cancer | 9 (9.7) | 10 (23.3) | 2.82 (1.05–7.58) | 0.034 |
| Organ transplant recipient | 1 (1.1) | 1 (2.3) | 2.19 (0.13–35.86) | 0.534 |
| Clinical presentation | ||||
| Severe sepsis/septic shock | 40/85 (47.1) | 31/42 (73.8) | 3.17 (1.41–7.12) | 0.004 |
| Nosocomial acquisition | 90 (96.8) | 42 (97.7) | 1.40 (0.14–13.86) | 0.773 |
| Source of infection | ||||
| Pneumonia* | 50 (53.8) | 26 (60.5) | 1.28 (0.61–2.68) | 0.504 |
| Tracheobronchitis | 16 (17.2) | 4 (9.3) | 0.49 (0.15–1.57) | 0.226 |
| Primary bacteremia | 6 (6.5) | 1 (2.3) | 0.34 (0.04–2.96) | 0.311 |
| Intrabdominal | 5 (5.4) | 4 (9.3) | 1.80 (0.46–7.08) | 0.463 |
| Skin and skin structures | 5 (5.4) | 4 (9.3) | 1.80 (0.46–7.08) | 0.463 |
| Catheter-related | 3 (3.2) | 3 (7) | 2.25 (0.43–11.63) | 0.380 |
| Meningitis/ventriculitis | 2 (2.2) | 0 | – | 1 |
| Urinary tract | 2 (2.2) | 1 (2.3) | 1.08 (0.09–12.28) | 1 |
| Others | 1 (1.1) | 0 | – | 1 |
| ST2 isolates | 59/92 (64.1) | 29 (67.4) | 1.15 (0.53–2.49) | 0.707 |
| ST79 isolates | 21/92 (22.8) | 3 (7) | 0.25 (0.07–0.90) | 0.025 |
| Sporadic isolates | 9/92 (9.8) | 3 (7) | 0.69 (0.17–2.69) | 0.594 |
| Appropriate antimicrobial therapy | 62 (66.6) | 16 (37.2) | 0.29 (0.13–0.63) | 0.002 |
| Combined treatment | 33/92 (35.9) | 9 (20.9) | 0.47 (0.20–1.10) | 0.081 |
| Mechanical ventilation | 73 (78.5) | 33 (76.7) | 0.90 (0.38–2.14) | 0.819 |
* VAP survivors n=41, death n=21.
The present study provides updated information about the clinical and molecular epidemiology of A. baumannii in Spanish ICU, allowing a suitable comparison with the situation ten years before, and identifying clinical and molecular variables associated with mortality in critically ill patients infected with A. baumannii. In this issue, our results highlight the positive impact on the outcome of appropriate empirical therapy in infections caused by A. baumannii and the importance of molecular epidemiology because ST79 clone is associated with lower mortality.
An outstanding finding is that the incidence density rate of A. baumannii has decreased markedly perhaps reflecting the improvement of the infection control measures in Spanish ICU.22 A drop of A. baumannii isolation in the ICU with a concomitant increment of its isolation in medical wards has also been reported.23 Respiratory infections (pneumonia or tracheobronchitis) are by far the most frequent presentation of A. baumannii infection in critically ill patients.
The profile of antimicrobial agents used to treat A. baumannii infections have changed in accordance with the pattern of resistances. Colistin is currently the most frequently administered antimicrobial agents whereas the use of carbapenems has markedly decreased in comparison with the year 2000 reflecting the current high rate of resistance. Nonetheless, it is outstanding that colistin was not used empirically. The concern about its toxicity and the lack of clinical trials evaluating the empirical use of this antimicrobial could explain this finding. Furthermore, susceptibility to aminoglycosides remained stable in both study periods. However, the use of this group of antibiotics decreased significantly in the second period both in the empirical therapy and in the definitive treatment. Possibly, the extensive use of colistin explained the decrease of aminoglycoside use to avoid the concomitant administration of two nephrotoxic agents.24
Almost 30% of the patients were treated with tigecycline in 2010, a new antimicrobial not available in the previous period with a mortality rate similar to that observed in infected patients treated with other agents. Recent meta-analyses have suggested an increased risk of death in patients receiving tigecycline compared to other antibiotics particularly in ventilator-associated pneumonia.25,26 The low number of cases and the fact that tigecycline was almost always used in combination therapy impedes us to draw valid conclusions regarding the likely excess of mortality in patients treated with tigecycline for severe A. baumannii infections.
MLST is considered the gold standard method to investigate the population structure and global epidemiology of A. baumannii.27 With this technique, we have detected the occurrence of profound changes of the genotypes in this 10-year period.10 Episodes of infection or colonization in Spanish ICU were caused by the spread of strains belonging to few genotypes, in particular ST2 (almost three of each four isolates in the ICU belong to this clone) and, to a lesser extent, ST79. The presence of ST2 clonal group in the ICU increased significantly in 2010 in comparison to the previous period. Other studies have documented that the genotype ST2 is the most commonly isolated clone in Mediterranean countries.6,28,29 It is isolated more frequently in the ICU than in the general wards and the rate of carbapenem resistance is higher than with other clones.30 Conversely, ST79 is an emerging clone recently reported in Spain and its resistance profile has not been clearly established.6 We found that the ST79 clone is resistant to the majority of the antimicrobials including a high rate of resistance to carbapenems.
In the unadjusted analysis for mortality in infected patients, underlying conditions such as chronic renal insufficiency or cancer were more frequently present in patients who died. A. baumannii is more common and causes more severe diseases among critically ill patients with severe comorbid conditions.3 Of note, appropriate antimicrobial therapy and ST79 clonal group were protective factors for mortality in the multivariate analysis.31
A. baumannii genotype influences the clinical presentation and outcome. ST79 clone is less frequently associated with severe sepsis or septic shock and with a lower mortality rate. After adjusting for confounding variables, ST79 clone and appropriate empirical therapy are protective factors of mortality in patients with A. baumannii infections. Carbapenem resistance or the rate of appropriate therapy cannot explain the lower mortality associated with the ST79 clone. Differences in virulence may provide further explanation for the different outcomes observed in infections caused by the different clones. In fact, strains assigned to genotypes ST2 exhibit a high virulence and a great capacity to produce biofilms.32 The impact on the outcome of the different A. baumannii clones patients has not been extensively assessed. Using REP-PCR, clone group (the most virulent group vs. the reference group) was an independent predictor of 14-day mortality in a case-control study of patients with carbapenem-resistant A. baumannii bacteremia.9
Furthermore, appropriate antimicrobial therapy was a protective factor for mortality.33,17,34 In our series, the use of combination therapy in critically ill patients with A. baumannii infection is not associated with lower mortality. Two clinical trials do not support the beneficial effect of combined treatment in A. baumannii infections (mainly VAP).35,36 An observational study that included patients (in the ICU and in wards) with A. baumannii infection in the 2010 cohort also failed to demonstrate clinical benefit of combination therapy.37 Nevertheless, a recent systematic review concludes that combination is superior to monotherapy in severely ill patients with A. baumannii infections.38
Diverse limitations of these two cohorts have been previously recognized.11 We also admit additional weaknesses of this sub-analysis. First, because the sample size of ICU patients was relatively small for some comparisons, a type II error is possible and studies with larger study population might demonstrate additional differences. Second, doses of the antimicrobials were not standardized. Moreover, a loading dose of colistin and the current dosages to optimize its antimicrobial properties were not used.39 These factors could also influence our clinical results. Third, severity of illness assessed by APACHE II score or any other validated scale was not recorded and it may alter the findings of the mortality analysis. Conversely, the prospective design, the strict and pre-defined criteria to differentiate infection from colonization, the reference techniques used to identify A. baumannii, and the novelty of the introduction of clonal type in the mortality analysis constitute strengths of the present research.
In summary, after 10 years of the first analysis, it is noteworthy a significant reduction of the incidence of A. baumannii isolation in Spanish ICU although without changes in mortality. As occurs in other European countries, ST2 clone was more frequently isolated in ICU patients. The antimicrobial susceptibility has also changed considerably being colistin almost the only antimicrobial active against all the A. baumannii isolates. Epidemic ST79 clonal group and appropriate empirical treatment are associated with a better prognosis. Our results do not support the use of combination therapy in critically ill patients with A. baumannii infections. These findings should be taken into account for the selection of empirical therapy in critically ill patients with high suspicion of A. baumannii infection.
Authors’ contributionsJGM was responsible for the conception, fund raising, design and coordination of the study; made substantial contributions to data acquisition, analysis and interpretation, and drafted the manuscript. AGP carried out the statistical analysis and made substantial contributions to interpretation of data. ADM made substantial contributions to analysis and interpretation of data. MEC, EG, CRA, FFC, JV, LMM, MTC, AP and GB made substantial contributions to the microbiological determinations. JPD, JMC, JRB made substantial and useful suggestions. All authors have revised the manuscript and approved the final version of manuscript.
Conflict of interest statementThe authors declare that they have no conflict of interest.
Supported by Ministerio de Economía y Competitividad, Instituto de Salud Carlos III (ISCIII) – co-financed by European Development Regional Fund “A way to achieve Europe” ERDF, Spanish Network for the Research in Infectious Diseases (REIPI RD12/0015). M. Tomás was financially supported by the Miguel Servet Program (C.H.U.A. Coruña and ISCIII).
The study was funded by Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III (FIS PI10/00056).
We are grateful to the following organizations and researchers who participated in the study: Virgen Rocío (Juan Antonio Márquez-Vácaro); Hospital Marqués de Valdecilla (Carmen Fariñas); Hospital SAS La Línea (Antonio Sánchez-Porto, Gloria Esteban Meruendano, Luis Barbeyto-Vales, Javier Casas-Ciria, Luis Vallejo); Complejo hospitalario de Ourense (Begona Fernández-Pérez, José Carlos Villar-Chao); Hospital Gregorio Maranón (Belén Padilla-Ortega, Emilia Cercenado-Mansilla); Hospital de Navarra (José Javier García-Irure); Hospital Costa del Sol-Marbella (Alfonso del Arco Jiménez); Hospital General de Valencia (Concepción Gimeno-Cardona, Juan Carlos Valía, Núria Tormo-Palop, Vicente Abril, Josefina Rifa, Maria Jesus Martinez-Garcia); Consorci Hospitalari de Vic (Joseph Vilaró-Pujals, Marian Navarro Aguirre, Ana Vilamala); Policlínica Guipúzkoa (José Antonio Jiménez-Alfaro, Carlos Reviejo-Jaca); Hospital Puerta del Mar (Pilar Marín Casanova, Francisca Guerreo, Evelyn Shaw, Virginia Plasencia); Complejo Hospitalario de Soria (Teresa Nebreda-Mayoral, María José Fernández-Calavia, Susana García de Cruz, Carmen Aldea-Mansilla); Hospital Universitario de Alicante (Esperanza Merino de Lucas, Alfredo Zorraquino, Sergio Reus-Bañuls); Hospital Infanta Cristina (Eugenio Garduno-Eseverri, Luis López Sánchez); Hospital Universitario Central de Asturias (Ana Fleites-Gutiérrez, Azucena Rodríguez-Guardado, Alfonso Moreno); Hospital Donostia (José María García-Arenzana Anguera); Complejo Hospitalario Torrecárdenas (Serafín López-Palmero, Manuel Rodríguez-Maresca); Complejo Hospitalario Xeral-Calde Lugo (Fernando García-Garrote, José Varela-Otero, María del Pilar Alonso); Hospital Universitario Reina Sofía de Córdoba (Elisa Vidal-Verdú, Fernando Rodríguez-López); Hospital Universitario Santiago Compostela (Fernanda Pardo-Sánchez, E. Ferrer-Vizoso, B. Regueiro-Garcia); Hospital Sant Pau (Mercé Gurgui, Roser Pericas, Virginia Pomar); Hospital Galdakao-Usansolo (Pedro María Olaechea-Astigarraga, Rafael Ayarza-Igartua); Hospital Son Dureta (María Dolores Maciá-Romero, Enrique Ruiz de Gopegui-Bordes); Hospital Puerta de Hierro (María Isabel Sánchez-Romero); Hospital Juan Grande (Jesús García-Mata, María José Goyanes, Cristina Morales-Mateos); Hospital San Cecilio (José Hernández-Quero, Trinidad Escobar-Lara); Hospital Sant Joan de Reus (Frederic Ballester-Bastardie, Simona Iftimie, Isabel Pujol-Bajador); Hospital de Motril (María Isabel Galán-Navarro, María Luz Cádiz-Gurrea); Hospital San Agustín (Carmen Amores-Antequera, Montserrat Gómez, Purificación Cantudo); Hospital de Granollers (Carmina Martí-Salas, Jordi Cuquet-Peragosa, Antonio Moreno-Flores, Luis Anibarro-García); Hospital de Segovia (Susana Hernando-Real, Pablo A. Carrero-González); Complejo Hospitalario de Pontevedra (María Angeles Pallarés-González, Sergio Rodríguez-Fernández); Hospital de Bellvitge (Miquel Pujol-Rojo, Fe Tubau); Hospital Virgen de la Victoria de Málaga (Enrique Nuno-Alvarez, María Ortega-Torres); Hospital Doctor Moliner (Salvador Giner-Almaraz, María Rosa Roca-Castelló, Manuela Castillo, Elena Hortelano); Hospital 12 de Octubre (Fernando Chaves-Sánchez, Ana García-Reyne); Hospital delMar (Juan Pablo Horcajada-Gallego, Concha Segura); Hospital San Agustín de Avilés (Gema Sierra-Dorado, Raquel Yano-Escudero); Complejo Hospitalario Materno Insular de Gran Canaria (María Elena Dorta-Hung, Cristóbal del Rosario Q).