To analyze the profile of antimicrobial susceptibility of meningococcal disease isolates collected throughout Brazil from 2006 to 2008 and forwarded to the National Reference Laboratory for Meningitis, Institute Adolfo Lutz - São Paulo.
Materials and methodsThe MIC to penicillin, ampicillin, chloramphenicol, ceftriaxone, ciprofloxacin and rifampicin was determined in a sample of 1096 (55% of the total isolates received) randomly chosen using the broth microdilution procedure. The breakpoints used were those recommended by the European Monitoring Group on Meningococci (EMGM).
ResultsDecreased susceptibility to penicillin and ampicillin was detected in 13% and 12.9% respectively. All isolates were susceptible to chloramphenicol, ceftriaxone, and ciprofloxacin. Two strains (0.2%) showed high resistance to rifampicin and 0.5% of the isolates displayed intermediate resistance to rifampicin.
ConclusionsThe meningococcal strains isolated in Brazil during 2006-2008 were globally susceptible to all antibiotics currently used in treatment or chemoprophylaxis of meningococcal disease in Brazil.
Analizar el perfil de susceptibilidad a los antimicrobianos de las cepas de meningococos aisladas de casos de enfermedad meningocócica en Brasil entre 2006 y 2008 y enviadas al Laboratorio Nacional de Referencia para Meningitis, Instituto Adolfo Lutz, São Paulo.
Material y métodosSe determinó la CIM a penicilina, ampicilina, cloranfenicol, ceftriaxona, ciprofloxacino y rifampicina, mediante el procedimiento de microdilución seriada en caldo en una muestra de 1.096 aislados (55% de los aislados recibidos) escogida al azar. Los puntos de corte utilizados fueron los recomendados por el European Monitoring Group on Meningococci (EMGM).
ResultadosSe detectó disminución de la susceptibilidad a la penicilina y la ampicilina en el 13 y el 12,9% respectivamente. Todos los aislados fueron susceptibles a cloranfenicol, ceftriaxona y ciprofloxacino. Dos cepas (0,2%) mostraron alta resistencia a la rifampicina y el 0,5% de los aislados presentaron resistencia intermedia a la rifampicina.
ConclusionesLas cepas de meningococos aisladas en Brasil en el periodo 2006-2008 fueron globalmente susceptibles a los antibióticos actualmente utilizados en el tratamiento o quimioprofilaxis de enfermedad meningocócica en Brasil.
Neisseria meningitidis is a pathogen of great public health importance for causing periodic epidemics of meningococcal disease with high case-fatality rate even with appropriate treatment and intensive care, affecting particularly infants, adolescents and young adults. Neisseria meningitidis is a leading cause of bacterial meningitis in Brazil, presenting, in the last ten years, a case-fatality rate of about 11% in its clinical form of meningitis, 16% in meningitis and septicemia, rising to 38% in septicemia. The clinical forms of meningitis, meningitis and septicemia and septicemia alone accounted for 39.2%, 33% and 27.8%, respectively, of the confirmed cases of meningococcal disease in Brazil, during this period. (http://www.saude.gov.br/sinanweb)
In view of the rapid progression and high lethality of the meningococcal disease, prompt treatment seems to be crucial to reduce the mortality and to improve the meningococcal disease outcome.
Penicillin remains as suitable treatment for meningococcal disease; however, since 1985 the increase of the meningococcal strains with reduced susceptibility to penicillin has been described in some countries, reaching percentages as 4% in the United States, 23% in Sweden, 30% in France and 70% in Turkey.1 Given the empiric therapy of patients with suspected bacterial meningitis and the high levels of resistance of Streptococcus pneumoniae to penicillin (about 30% in Brazil),2 initial therapy is based on the third-generation cephalosporin, such as ceftriaxone and cefotaxime in industrialized countries.3–5 Meningococcal strains with reduced susceptibility to ceftriaxone have not been described. However, the description of Neisseria gonorrhoeae strains resistant to cefixime, also a third-generation cephalosporin, associated with alterations in penA gene, alerts for the possibility of this resistance to be extended to meningococci in the future.4 Chloramphenicol has not been used for treatment of meningococcal disease in many countries for the description of its toxic effect, as the marrow aplasia.6 High-level chloramphenicol resistance was described in Vietnam and in France.7 The rifampicin and ciprofloxacin are widely used as chemoprophylaxis of contacts of patients with meningococcal disease. Rifampicin resistance is not widely present and it is usually limited to sporadic cases. However, some reports on meningococcal resistance to ciprofloxacin might cause concern because the potential for spreading the resistance.8–13
The worldwide problem of resistance in N. meningitidis emphasizes the urgent need of careful surveillance on antibiotic susceptibilities for better control and prevention of meningococcal disease.
The aim of the present study was to describe the susceptibility profile to antimicrobials of a large collection of meningococcal isolated in Brazil during the period of 2006-2008.
Material and methodsBacterial strainsThe Institute Adolfo Lutz (IAL), located in the State of São Paulo, is the Brazilian National Reference Laboratory for Bacterial Meningitis, to where clinical isolates are forwarded through a national epidemiologic system. The IAL receives an annual average of 660 meningococcal invasive isolates from all Brazilian regions for full phenotypic characterization. Between 2006 and 2008, 7 720 cases of meningococcal disease were reported in Brazil of which 2 567 (33.3%) were laboratory-confirmed by culture. Of the 2 567 cases confirmed by culture, 1 988 strains (77.4%) were sent to the IAL by 27 Public Health Laboratories and hospitals located in the Southeast (59.2%), Northeast (17.6%), Central-West (8.8%), South (11.9%) and North (2.5%) regions of Brazil. From this collection, a statistical representative random sampling of 1 096 (55.1%) strains was submitted to antimicrobial susceptibility tests, calculated by using the Statcalc Program (Epi Info software version 6.04, Centers for Diseases Control and Prevention, Atlanta, GA). The isolates were recovered from cerebrospinal fluid (n=817; 74.5%) or blood (n=279; 25.5%).
The selected sample was comprised by serogroups B (n=344 [31.4%]), C (n=677 [61.8%]), W135 (n=60 [5.4%]), and Y (n=15 [1.4%]). Serogroup B isolates included 50 phenotypes being the most prevalent 4,7:P1.19,15 (220/344; 63.9%) and 4,7:P1.7,1 (24/344; 7%) with the remaining serogroup B phenotypes representing less than 2%. Serogroup C included 27 different phenotypes, being the most prevalent 23:P1.14-6 (560/677; 82.7%), and 2a:P1.5 (31/677; 4.6%) with the remaining serogroup C phenotypes representing less than 3%. Serogroup W135 included 14 phenotypes being the most prevalent 2a:P1.2 (19/60; 31.7%), 2a:P1.5 (10/60; 16.7%) and 2b:P1.2 (6/60; 10%) with the remaining serogroup W135 phenotypes representing less than 5%. Serogroup Y included 8 different phenotypes being the most prevalent 17,7:P1.5 (7/15; 46.7%) followed by 4,14:P1.7 (2/15; 13.3%) with the remaining serogroup Y phenotypes representing less than 7%.
Phenotypic antimicrobial susceptibility testingThe susceptibility of meningococcal strains to penicillin, ampicillin, chloramphenicol, ciprofloxacin, ceftriaxone and rifampicin was analyzed by determining the minimal inhibitory concentration (MIC) using the broth microdilution procedure described in CLSI document M7-A7.14,15 The susceptibility/resistance breakpoints were those recommended by the European Monitoring Group on Meningococci (EMGM),5 as follows: penicillin G ≤0.06/≥1μg/mL (Susceptible ≤0.06μg/mL, Intermediate=0.125-0.500μg/mL, Resistant ≥1μg/mL); ampicillin ≤0.12/≥2μg/mL (Susceptible ≤0.125μg/mL, Intermediate=0.250-1μg/mL, Resistant ≥2μg/mL); chloramphenicol ≤2/≥8μg/mL (Susceptible ≤2μg/mL, Intermediate=4μg/mL, Resistant ≥8μg/mL); ciprofloxacin ≤0.03/≥0.5μg/mL (Susceptible ≤0.03μg/mL, Intermediate=0.06-0.25μg/mL, Resistant ≥0.5μg/mL); ceftriaxone ≤0.12μg/mL (Susceptible ≤0.12μg/mL, ≥0.12μg/mL not described), and rifampicin ≤0.25/≥2μg/mL (Susceptible ≤0.25μg/mL, Intermediate=0.5-1μg/mL, Resistant ≥2μg/mL).
Analysis of the molecular mechanism of resistance to rifampicinA fragment of the rpoB gene (encoding amino acids 435–644) was amplified using primers rpoB-F1 and rpoB-R1as previously described.16
Quality controlAn external Quality Assurance Program for characterization (QAP), introduced by the SIREVA network in Latin American countries17 has validated the performance of the N. meningitidis typing and antimicrobial susceptibility tests. The QAP is coordinated by the Reference Laboratory for Neisserias, National Center for Microbiology, Institute of Health Carlos III, Majadahonda, Madrid, Spain.
Statistical analysisAssociation between antimicrobial resistance and variables such as age and gender of patients, N. meningitidis serogroup and the geographic distribution of isolates were assessed by the χ2 test; P values <.05 were considered to be statistically significant.
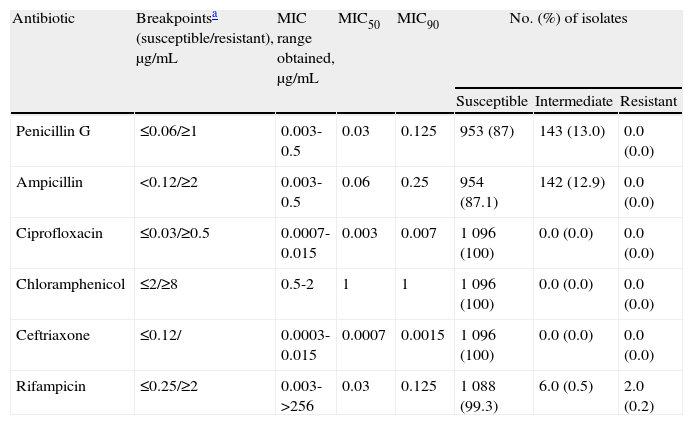
ResultsThe results of antimicrobial susceptibility testing are summarized in table 1 table 1. All isolates were susceptible to ciprofloxacin, ceftriaxone, and chloramphenicol. Ciprofloxacin and ceftriaxone were the antibiotics with higher inhibitory activities showing minimal MIC90 at the very low concentration range. MIC of 1μg/mL to chloramphenicol, a concentration very near to the intermediate category (4μg/mL) was detected in most strains (78%). There was a broad range of MICs to rifampicin, from 0.007μg/mL to 0.125μg/mL for most isolates. A total of 143 (13%) and 142 (12.9%) strains presented reduced susceptibility to penicillin and ampicillin, respectively. Although most of the isolates were susceptible to penicillin (63.9%) or to ampicillin (77.8%) far from the breakpoint values, 253 (23.1%) of them were only inhibited by 0.06μg/mL, a value close to the penicillin intermediate category (data not shown), suggesting a tendency to a raise in MICs to penicillin.
Susceptibility to six different antimicrobials of Neisseria meningitidis strains (n=1 096) isolated from invasive disease in Brazil from 2006 to 2008.
| Antibiotic | Breakpointsa (susceptible/resistant), μg/mL | MIC range obtained, μg/mL | MIC50 | MIC90 | No. (%) of isolates | ||
| Susceptible | Intermediate | Resistant | |||||
| Penicillin G | ≤0.06/≥1 | 0.003-0.5 | 0.03 | 0.125 | 953 (87) | 143 (13.0) | 0.0 (0.0) |
| Ampicillin | <0.12/≥2 | 0.003-0.5 | 0.06 | 0.25 | 954 (87.1) | 142 (12.9) | 0.0 (0.0) |
| Ciprofloxacin | ≤0.03/≥0.5 | 0.0007-0.015 | 0.003 | 0.007 | 1 096 (100) | 0.0 (0.0) | 0.0 (0.0) |
| Chloramphenicol | ≤2/≥8 | 0.5-2 | 1 | 1 | 1 096 (100) | 0.0 (0.0) | 0.0 (0.0) |
| Ceftriaxone | ≤0.12/ | 0.0003-0.015 | 0.0007 | 0.0015 | 1 096 (100) | 0.0 (0.0) | 0.0 (0.0) |
| Rifampicin | ≤0.25/≥2 | 0.003->256 | 0.03 | 0.125 | 1 088 (99.3) | 6.0 (0.5) | 2.0 (0.2) |
MIC: minimum inhibitory concentration; MIC50 and MIC90: minimum inhibitory concentration at which respectively 50% and 90% of isolates were inhibited.
Two isolates belonging to phenotypes B:4,7:P1.19,15 and C:23:P1.14-6 presented high resistance (MIC>256μg/mL) to rifampicin (0.2%), and six (0.5%) isolates showed intermediate resistance to rifampicin. The analysis of the molecular mechanism of rifampicin resistance of the B:4,7:P1.19,15 and C:23:P1.14-6 isolates showed only one amino acid substitution: a Hys>Tyr, in position 552 of the rpoB gene. Both strains showed not only the same amino acid substitution but also the same rpoB allele based in the DNA sequence.
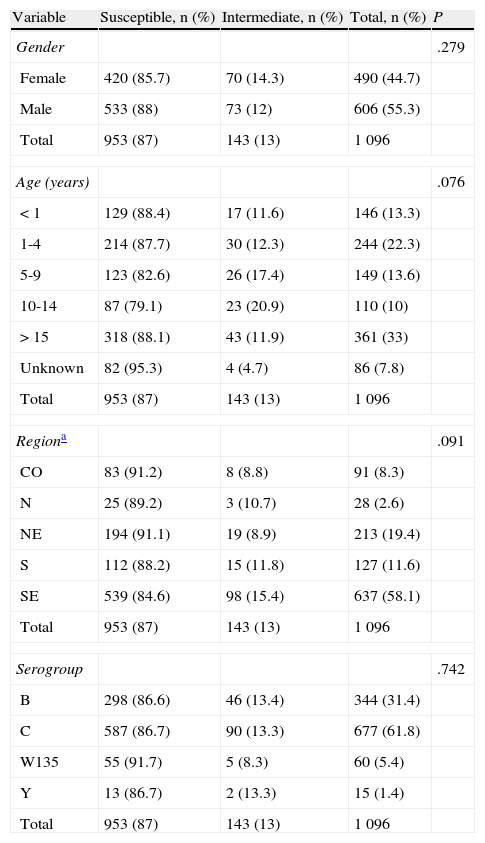
In order to better understand the distribution of the strains with reduced susceptibility to penicillin in the studied sample, we correlated the susceptibility with some variables as shown in table 2 table 2. There was no statistically significant association between the diminished susceptibility to penicillin and gender (P=.279), age (P=.076), geographic region (P=.091), or serogroup of N. meningitidis (P=.742).
Susceptibility of Neisseria meningitidis strains (n=1 096) to penicillin, according to patient characteristics, geographic regions and serogroups, Brazil from 2006 to 2008.
| Variable | Susceptible, n (%) | Intermediate, n (%) | Total, n (%) | P |
| Gender | .279 | |||
| Female | 420 (85.7) | 70 (14.3) | 490 (44.7) | |
| Male | 533 (88) | 73 (12) | 606 (55.3) | |
| Total | 953 (87) | 143 (13) | 1 096 | |
| Age (years) | .076 | |||
| < 1 | 129 (88.4) | 17 (11.6) | 146 (13.3) | |
| 1-4 | 214 (87.7) | 30 (12.3) | 244 (22.3) | |
| 5-9 | 123 (82.6) | 26 (17.4) | 149 (13.6) | |
| 10-14 | 87 (79.1) | 23 (20.9) | 110 (10) | |
| > 15 | 318 (88.1) | 43 (11.9) | 361 (33) | |
| Unknown | 82 (95.3) | 4 (4.7) | 86 (7.8) | |
| Total | 953 (87) | 143 (13) | 1 096 | |
| Regiona | .091 | |||
| CO | 83 (91.2) | 8 (8.8) | 91 (8.3) | |
| N | 25 (89.2) | 3 (10.7) | 28 (2.6) | |
| NE | 194 (91.1) | 19 (8.9) | 213 (19.4) | |
| S | 112 (88.2) | 15 (11.8) | 127 (11.6) | |
| SE | 539 (84.6) | 98 (15.4) | 637 (58.1) | |
| Total | 953 (87) | 143 (13) | 1 096 | |
| Serogroup | .742 | |||
| B | 298 (86.6) | 46 (13.4) | 344 (31.4) | |
| C | 587 (86.7) | 90 (13.3) | 677 (61.8) | |
| W135 | 55 (91.7) | 5 (8.3) | 60 (5.4) | |
| Y | 13 (86.7) | 2 (13.3) | 15 (1.4) | |
| Total | 953 (87) | 143 (13) | 1 096 | |
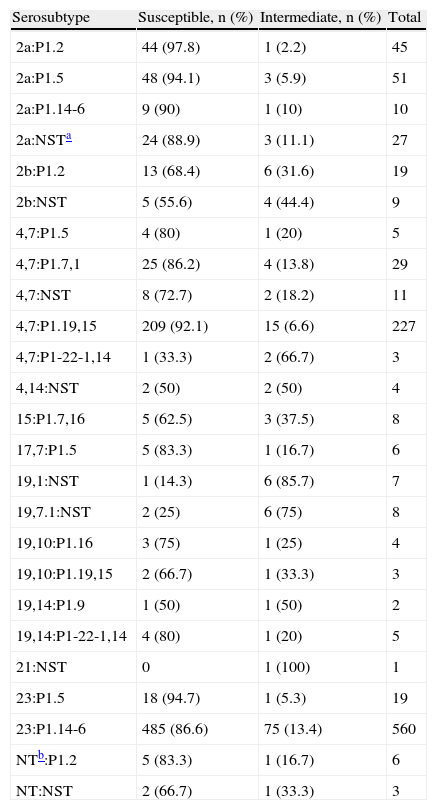
The isolates with reduced susceptibility to penicillin belonged to a large variety of serosubtypes as shown in table 3 table 3. The phenotypes 4,7:P1.19,15 and 23:P1.14-6 were the most prevalent among serogroup B and C isolates, respectively; however, the prevalence of isolates with reduced sensibility to penicillin was not particularly associated with any serosubtype (data not submitted to statistical analysis by serosubtype diversity).
Serosubtype distribution among Neisseria meningitidis strains displaying reduced susceptibility to penicillin, Brazil from 2006 to 2008.
| Serosubtype | Susceptible, n (%) | Intermediate, n (%) | Total |
| 2a:P1.2 | 44 (97.8) | 1 (2.2) | 45 |
| 2a:P1.5 | 48 (94.1) | 3 (5.9) | 51 |
| 2a:P1.14-6 | 9 (90) | 1 (10) | 10 |
| 2a:NSTa | 24 (88.9) | 3 (11.1) | 27 |
| 2b:P1.2 | 13 (68.4) | 6 (31.6) | 19 |
| 2b:NST | 5 (55.6) | 4 (44.4) | 9 |
| 4,7:P1.5 | 4 (80) | 1 (20) | 5 |
| 4,7:P1.7,1 | 25 (86.2) | 4 (13.8) | 29 |
| 4,7:NST | 8 (72.7) | 2 (18.2) | 11 |
| 4,7:P1.19,15 | 209 (92.1) | 15 (6.6) | 227 |
| 4,7:P1-22-1,14 | 1 (33.3) | 2 (66.7) | 3 |
| 4,14:NST | 2 (50) | 2 (50) | 4 |
| 15:P1.7,16 | 5 (62.5) | 3 (37.5) | 8 |
| 17,7:P1.5 | 5 (83.3) | 1 (16.7) | 6 |
| 19,1:NST | 1 (14.3) | 6 (85.7) | 7 |
| 19,7.1:NST | 2 (25) | 6 (75) | 8 |
| 19,10:P1.16 | 3 (75) | 1 (25) | 4 |
| 19,10:P1.19,15 | 2 (66.7) | 1 (33.3) | 3 |
| 19,14:P1.9 | 1 (50) | 1 (50) | 2 |
| 19,14:P1-22-1,14 | 4 (80) | 1 (20) | 5 |
| 21:NST | 0 | 1 (100) | 1 |
| 23:P1.5 | 18 (94.7) | 1 (5.3) | 19 |
| 23:P1.14-6 | 485 (86.6) | 75 (13.4) | 560 |
| NTb:P1.2 | 5 (83.3) | 1 (16.7) | 6 |
| NT:NST | 2 (66.7) | 1 (33.3) | 3 |
The data show that meningococcal strains isolated in Brazil during 2006-2008 were globally susceptible to all antibiotics currently used both in treatment or chemoprophylaxis of meningococcal disease in the country.
Although fluoroquinolones have been recommended for chemoprophylaxis especially in adults for their efficacy in eradicating the meningococcus from the nasopharynx in 97% of the individuals for up to 33 days after the end of therapy, in a single-dose regimen18 and resistance has been already described in different geographical locations including Latin American region.12,13,19–22
The reduced susceptibility to ceftriaxone has not been described around the world, and was not observed in Brazilian meningococcal strains to which ceftriaxone showed very high inhibitory activities, with MIC90 within the very low concentration range.
No resistance to chloramphenicol was observed, but MIC of 1μg/mL, which is very near to the intermediate category (4μg/mL), was obtained for most of the isolates. This drug therefore can be an effective antibiotic therapy substitute, particularly in penicillin-allergic patients.
In spite of the fact that rifampicin has been widely used in Brazil for preventing secondary cases, in the present study N. meningitidis isolates remained highly susceptible to this antimicrobial drug (only two resistant isolates, 0.2%), as it has been described by others.16,23–26 The analysis of the molecular mechanism conferring resistance to rifampicin of both isolates showed a point mutation in a specific region of the rpoB gene as was already found in two strains from Spain.8 Our results support the use of rifampicin prophylaxis of meningococcal infections in Brazil, paralleled with a monitoring susceptibility program. A data base with alleles of the rpoB fragment analyzed in this study is under construction on the European Meningococcal Disease Society (EMGM) hosted on the web page http://www.neisseria.org which will allow the comparison among strains showing different level of rifampicin resistance and will be helpful to define proper breakpoints to be used for this antimicrobial drug.
In our study, penicillin-intermediate susceptibility isolates displayed a uniform distribution regarding to age, gender or geographic region. The prevalence of meningococcal strains with reduced susceptibility to penicillin remained stable over the analyzed period (2006-2008), ranging from 10.3% to 15.1% (data not shown). However, when these data are compared to the previous period of 2000-2005,27 the prevalence of meningococci with reduced susceptibility to penicillin is shown to be clearly increasing from 0 to 15.1% over the 9-years period, ranging from 0% of penicillin-intermediate susceptibility isolates in 2000, to 4.1% in 2001, 3.1% in 2002, 4.1% in 2003, 6.2% in 2004, 8.9% in 2005, 13.5% in 2006, 10.3% in 2007, and 15.1% in 2008. The increase of the prevalence of meningococci with reduced susceptibility to penicillin seen in the period 2000-2008 was paralleled by a shift in the serogroup distribution. Serogroup B meningococci, B:4,7:P1.19,15-ST-5 complex ET-5 was hyperendemic in Brazil since 1988, accounting for 80% of all meningococcal disease between 1988 and 1989.28,29 However, there was a huge change in the prevalence of the serogroups with the increasing prevalence of a single serogroup C clone, C:23:P1.14-6, ST-103 Clonal Complex. This clone, which emerged in Brazil in 1990, has been gradually increasing, becoming the most prevalent phenotype in 2003, and accounting for 67.7% of N. meningitidis isolates in 2008.30
These data are in agreement with those from other countries such as Italy where the prevalence of penicillin-intermediate susceptibility isolates has significantly risen from 7.5% before 2002 to 27.4% in 2004 being directly associated with an increase in serogroup C (C:2b:P1.5) strains 31, or Spain where an increase of penicillin-intermediate susceptibility isolates from 0.4% to 43% in only 5 years (1985-1990) was reported and associated with a shift between meningococcal serogroups B (B:4:P1.15) and C (C:2b:P1.5,2) isolates.9,31,32 However, the penicillin-intermediate susceptibility isolates distribution has not been associated to meningococcal serogroups or serosubtypes in this study and the prevalence increase of isolates with reduced susceptibility to penicillin can not be associated with the emergence of clone C:23:P1.14-6, ST-103 complex in Brazil. These results suggest that the rising prevalence of meningococcal penicillin-intermediate susceptibility strains is most probably due to a selective pressure created by the wide use of antimicrobial drugs, resulting in novel alleles that are mosaic genes encoding proteins with decreased affinity for penicillin, instead of the emergence and spread of a single clone exhibiting reduced susceptibility to penicillin. These results are in agreement with those of South Africa, where penicillin resistance do not appear to be due to the spread of a single clone.1
Although the prevalence of strains with reduced penicillin susceptibility in Brazil is not so high to be an alarming question, since the alteration of only one of the N. meningitidis PBPs (PBP2) has been described until now, strains with decreased susceptibility might be still evolving around the world. Therefore, the emergence of penicillin-resistant strains may be expected in the future indicating the need of a continued surveillance for monitoring the trends on antimicrobial susceptibility in N. meningitidis.
Conflict of interestThe authors have no conflicts of interest to declare.
We thank the central laboratory of each Brazilian State for providing the meningococcal isolates; Conceição Martins da Costa Zanelato and Marta Galhardo for identification and sorogrouping the isolates; Samanta Cristine Grassi Almeida for excellent technical assistance on MIC; Dra. Maria Regina Alves Cardoso and Dra. Denise Pimentel Bergamaschi for excellent statistical assistance.