Gonorrhoea remains an important health problem worldwide. The latest European guidelines have recommended the introduction of dual antimicrobial therapy due to the increase in its resistance to antimicrobial agents.
MethodsIn the present study, the susceptibility to some antibiotics was evaluated, and the typing of Neisseria gonorrhoeae strains was also performed. All Neisseria gonorrhoeae (NG) strains isolated from January 2012 to October 2014 were included in this work. Gonococcal isolates were tested for susceptibility according to the recommendations of both the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST). A total of 65 isolates were typed by the NG multi-antigen sequence types (NG-MAST) technique.
ResultsThe most frequent types found were ST 1407, ST 5405, ST 2992, and ST 5120. If CLSI and EUCAST criteria were applied, an ST 9807 type was found non-susceptible to ceftriaxone and cefixime (MIC 0.5μg/mL). When only EUCAST breakpoints were taken into account, three strains were also resistant to cefixime (MIC 0.25μg/mL) and two isolates were resistant to ceftriaxone (MIC 0.19 and 0.25μg/mL, respectively). The majority of strains were resistant to ciprofloxacin, and all Neisseria gonorrhoeae strains were susceptible to spectinomycin; twenty-five percent of isolates were resistant to azithromycin.
ConclusionsThe implementation of antimicrobial surveillance programs at regional level should be part of an overall gonococcal infection control strategy. Efforts should be made to perform antimicrobial susceptibility, a “cured test” in all gonorrhoea cases, and identify treatment failures to verify emerging resistance. Some types have been associated with decreased susceptibility to cephalosporins, making molecular typing a useful tool to predict antimicrobial resistance.
La gonorrea sigue siendo un importante problema de salud pública a nivel mundial. Las últimas guías en Europa han recomendado la introducción de tratamiento antimicrobiano dual debido al incremento de las resistencias a los antimicrobianos.
MétodosEn este estudio fue evaluada la sensibilidad a algunos antibióticos y se realizó el tipado de cepas de Neisseria gonorrhoeae. Todas las cepas aisladas desde enero de 2012 hasta octubre de 2014 fueron incluidas en este trabajo. El estudio de la sensibilidad de las cepas de gonococo fue realizado según las recomendaciones del Clinical and Laboratory Standards Institute (CLSI) y del European Committee on Antimicrobial Susceptibility Testing (EUCAST). Un total de 65 cepas fueron tipadas mediante la técnica NG-multiantigen sequence types (NG-MAST).
ResultadosLos tipos encontrados más frecuentemente fueron el ST 1407, ST 5405, ST 2992 y el ST 5120. Al aplicar los criterios del CLSI y del EUCAST, un tipo ST 9807 resultó no sensible a ceftriaxona y cefixime (CMI 0,5μg/mL). Al tener solo en cuenta los puntos de corte del EUCAST, 3 cepas más fueron también resistentes a cefixime (CMI 0,25μgr/mL) y 2 más fueron resistentes a ceftriaxona (CMI 0,19 y 0,25μg/mL, respectivamente). La mayoría de las cepas fueron resistentes a ciprofloxacino, y todas las cepas testadas fueron sensibles a espectinomicina; el 25% de los aislamientos fueron resistentes a azitromicina.
ConclusionesLa implementación de programas de vigilancia antimicrobiana a nivel regional debe ser parte de la estrategia de control para la infección gonocócica. Deben ser realizados esfuerzos para el estudio de la sensibilidad antimicrobiana, test de cura en todos los casos de gonorrea y la identificación de los fallos de tratamiento para verificar el aumento de resistencias. Algunos tipos han sido asociados con disminución de la sensibilidad a cefalosporinas, por lo que el tipado molecular puede ser una herramienta diagnóstica útil para predecir la resistencia a antibióticos.
Gonorrhoea is the second most commonly reported bacteial disease causing sexually transmitted infections (STIs) worldwide.1 The prevalence of this infection varies among populations but it continues to present a serious public health problem in many countries.2 Thus, an appropriate diagnosis and an effective treatment of this infection are important factors contributing to public health control and to prevent serious complications.
Treatment is usually given empirically and it is based on national or regional guidelines. Currently, third generation cephalosporins such as ceftriaxone or cefixime are the treatments of choice for this infection. However, the increase of resistance to recommended treatments for gonorrhoea may seriously affect infection control.3
Recently, some authors have described emergence of isolates exhibiting decreased susceptibility and resistance to the third generation cephalosporins which have caused treatment failure in gonococcal infections.4,5 In 2010, 9% of isolates from European countries showed decreased susceptibility to cefixime, increase in minimum inhibitory concentrations (MICs) for ceftriaxone, and a high prevalence of resistance to ciprofloxacin and azithromycin.
Due to these facts, antimicrobial surveillance programs are necessary to detect patterns of resistance not only at international and national levels, but also at regional level to ensure treatment efficacy against this infection. In this sense, molecular epidemiology surveillance can help to provide additional information on the emergence and dissemination of antimicrobial resistance. Molecular studies for epidemiological surveillance are necessary to detect association between genotype and antimicrobial resistance and to know how resistant strains emerge and disseminate.6
The objective of the present study was to analyze the antimicrobial susceptibility of Neisseria gonorrhoeae (NG) strains isolated from genital specimens in patients belonging to the health area of the Hospital of Poniente, as well as to perform the genotyping of these samples in order to detect epidemiological clusters of infection and possible modifications of the susceptibility patterns.
MethodsCollection of NG strainsAll NG strains isolated from January 2012 to October 2014 were included in this study. The genital samples were obtained from patients with STIs belonging to the health area of the Hospital of Poniente (El Ejido, Almería, Spain), and were sent to the microbiology laboratory for culture. A total of 65 isolates were included for both antimicrobial susceptibility study and molecular typing. All samples were obtained from genital sites (urethral or endocervical/vaginal exudates); fifty-one samples were taken from men. All samples were cultured in VCA agar (BioMérieux, France). The identification of NG suspected strains was performed by means of Gram stain, oxidase and catalase production, and finally with both biochemical analysis by means of the API NH system (BioMérieux, France) and Maldi-tof technology (Vitek MS, bioMérieux, France). The identification of all strains was confirmed at the National Centre of Microbiology (Instituto de Salud Carlos III, Madrid, Spain).
Antimicrobial susceptibility testingGonococcal isolates were tested for susceptibility according to the recommendations of Clinical and Laboratory Standards Institute (CLSI)7 and the European Committee on Antimicrobial Susceptibility Testing (EUCAST).8 All strains were tested for susceptibility to penicillin, ceftriaxone, cefixime, tetracycline, ciprofloxacin, azithromycin and spectinomycin by means of agar dilution tests. All isolates were also tested for penicillinase production using the Cefinase test (bioMérieux, Marcy-l’Etoile, France). MIC interpretation was performed according to both CLSI and EUCAST, and then compared.
Molecular typingAll NG strains (n=65) were typed by the NG multi-antigen sequence types (NG-MAST) technique,9 which differentiates strains on the basis of sequence variation in fragments of two hypervariable genes, the subunit B of the transferring binding protein (tbpB) and the porin Por B (porB). The typing was performed at the National Centre of Microbiology (Instituto de Salud Carlos III, Madrid, Spain). Allele numbers and STs were assigned using NG-MAST databases (www.ng-mast.net). Moreover, all NG strains were also serotyped at this center by means of the Phadebact® Monoclonal GC Test (MKL Diagnostics AB, Sollentuna, Sweden).
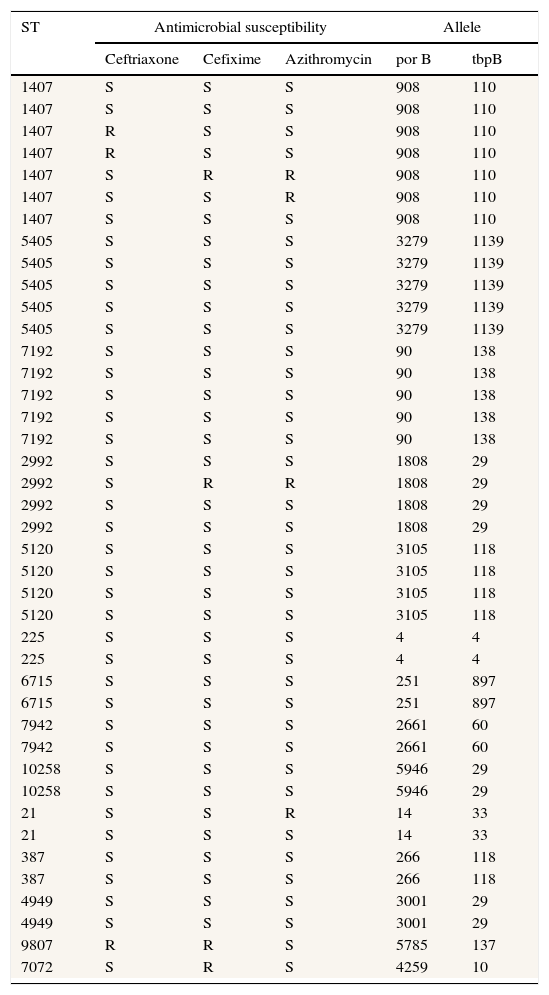
ResultsThe age of patients included in this study range from 21 to 47 years old (median age=32 years old). Twenty-eight samples were obtained from immigrant population and 37 from indigenous patients. The majority of strains were obtained from urethral samples (n=55) whereas 10 strains were obtained from vaginal or endocervical fluid. From the 65 typed strains, 34 different STs were obtained. The distribution of main types is shown in Table 1. The most frequent types found were STs 1407 (n=7), STs 5405 (n=5), STs 7192 (n=5), STs 2992 (n=4), and STs 5120 (n=4). An important finding is that a ST 9807 strain was nonsusceptible (according to CLSI and EUCAST criteria) to ceftriaxone and cefixime (MIC 0.5μg/mL for both antibiotics). Moreover, according to EUCAST criteria, three types were also resistant to cefixime (ST 2992, ST 7072 and ST 1407; MIC 0.25μg/mL) and two more types were resistant to ceftriaxone (ST 1407 in both cases; MIC 0.19 and 0.25μg/mL, respectively). All STs 1407 included in this study were ciprofloxacin resistant.
Main types of Neisseria gonorrhoeae strains (NG-MAST) related to the susceptibility to some antimicrobials (according to EUCAST).
| ST | Antimicrobial susceptibility | Allele | |||
|---|---|---|---|---|---|
| Ceftriaxone | Cefixime | Azithromycin | por B | tbpB | |
| 1407 | S | S | S | 908 | 110 |
| 1407 | S | S | S | 908 | 110 |
| 1407 | R | S | S | 908 | 110 |
| 1407 | R | S | S | 908 | 110 |
| 1407 | S | R | R | 908 | 110 |
| 1407 | S | S | R | 908 | 110 |
| 1407 | S | S | S | 908 | 110 |
| 5405 | S | S | S | 3279 | 1139 |
| 5405 | S | S | S | 3279 | 1139 |
| 5405 | S | S | S | 3279 | 1139 |
| 5405 | S | S | S | 3279 | 1139 |
| 5405 | S | S | S | 3279 | 1139 |
| 7192 | S | S | S | 90 | 138 |
| 7192 | S | S | S | 90 | 138 |
| 7192 | S | S | S | 90 | 138 |
| 7192 | S | S | S | 90 | 138 |
| 7192 | S | S | S | 90 | 138 |
| 2992 | S | S | S | 1808 | 29 |
| 2992 | S | R | R | 1808 | 29 |
| 2992 | S | S | S | 1808 | 29 |
| 2992 | S | S | S | 1808 | 29 |
| 5120 | S | S | S | 3105 | 118 |
| 5120 | S | S | S | 3105 | 118 |
| 5120 | S | S | S | 3105 | 118 |
| 5120 | S | S | S | 3105 | 118 |
| 225 | S | S | S | 4 | 4 |
| 225 | S | S | S | 4 | 4 |
| 6715 | S | S | S | 251 | 897 |
| 6715 | S | S | S | 251 | 897 |
| 7942 | S | S | S | 2661 | 60 |
| 7942 | S | S | S | 2661 | 60 |
| 10258 | S | S | S | 5946 | 29 |
| 10258 | S | S | S | 5946 | 29 |
| 21 | S | S | R | 14 | 33 |
| 21 | S | S | S | 14 | 33 |
| 387 | S | S | S | 266 | 118 |
| 387 | S | S | S | 266 | 118 |
| 4949 | S | S | S | 3001 | 29 |
| 4949 | S | S | S | 3001 | 29 |
| 9807 | R | R | S | 5785 | 137 |
| 7072 | S | R | S | 4259 | 10 |
S, susceptible; R, resistant.
All NG strains were serotyped, belonging to the IB serotype. Currently, studies of penA gene for ST 9807 ceftriaxone nonsusceptible strain are being performed.
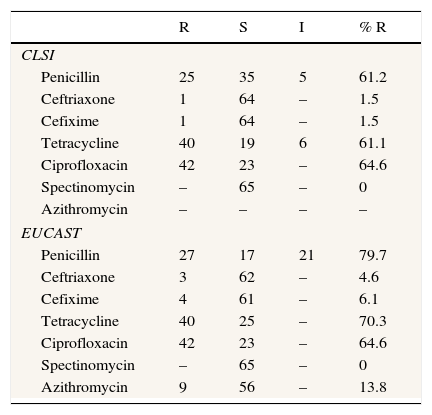
Ten isolates (18.5%) demonstrated penicillinase production (Cefinase test). If only CLSI criteria are applied, only a gonococcal strain (ST 9807) was nonsusceptible to ceftriaxone and cefixime, whereas according to EUCAST criteria four gonococcal strains were resistant to cefixime and three were resistant to ceftriaxone. According to EUCAST criteria, only 11 gonococcal strains were susceptible to penicillin, but if CLSI criteria are applied 21 isolates were susceptible to this antibiotic. The majority of isolates in this study were resistant to ciprofloxacin (n=42). All NG strains were susceptible to spectinomycin. With regard to azithromycin, from 65 strains tested for this antibiotic, 9 were resistant. The complete data corresponding to gonococcal strains susceptibility and resistance are shown in Table 2.
Antimicrobial susceptibility comparison between CLSI and EUCAST.
| R | S | I | % R | |
|---|---|---|---|---|
| CLSI | ||||
| Penicillin | 25 | 35 | 5 | 61.2 |
| Ceftriaxone | 1 | 64 | – | 1.5 |
| Cefixime | 1 | 64 | – | 1.5 |
| Tetracycline | 40 | 19 | 6 | 61.1 |
| Ciprofloxacin | 42 | 23 | – | 64.6 |
| Spectinomycin | – | 65 | – | 0 |
| Azithromycin | – | – | – | – |
| EUCAST | ||||
| Penicillin | 27 | 17 | 21 | 79.7 |
| Ceftriaxone | 3 | 62 | – | 4.6 |
| Cefixime | 4 | 61 | – | 6.1 |
| Tetracycline | 40 | 25 | – | 70.3 |
| Ciprofloxacin | 42 | 23 | – | 64.6 |
| Spectinomycin | – | 65 | – | 0 |
| Azithromycin | 9 | 56 | – | 13.8 |
CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing; R, resistant; S, susceptible; I, intermediate.
From 65 samples, only 7 strains were susceptible to all antibiotics tested; 3 strains were resistant to more than three antibiotics (multiresistant), 14 were resistant to three antibiotics, 7 resistant to two antibiotics and finally 23 were resistant to only one antibiotic.
With regard to isolates distribution in the health area of Hospital of Poniente, a concentration of strains and an epidemiological relationship could be observed. Three strains of ST1407 type, three strains of ST 5405 type, two strains of ST 2992 type and two strains of ST 5120 type are grouped and located in the same geographical area. Moreover, the strain ST 9807 (nonsusceptible to ceftriaxone and cefixime) was also located in the main epidemiological focus.
A patient presented in a period of a month two different isolates, ST 6715 and ST 4260. This patient presented one year later again with ST 6715 after correct treatment.
From the 20 strains belonging to types ST 1407, ST 5405, ST 2992, and ST 5120, twelve of them were found in immigrant population and these strains appear to have had epidemiological relationship.
During the follow-up period, test of cure was not carried out in the majority of patients (n=42), whereas test of cure was performed in 12 patients at least 72h after completion of treatment. From these patients the culture was negative in 11, but in one patient a new type of N. gonorrhoeae was found.
DiscussionGonorrhoea remains an important public health problem because untreated infections may lead to severe sequelae. In 2008, the World Health Organization (WHO) estimated 106 million cases among adults worldwide, although the true incidence is probably underestimated. Thus, effective treatment of gonorrhoea is crucial to public health control, but the progressive increase of resistance to recommended treatment regimens has compromised infection control efforts. Over the past decades, resistance has been identified to several antibiotics such as penicillin, tetracyclines and quinolones.10 The results of the present study also show a high percentage of resistance to these antibiotics such as penicillin [61.2% (CLSI); 79.7% (EUCAST)], tetracycline [61.1% (CLSI); 70.3% (EUCAST)], and ciprofloxacin (64.6%), so these antimicrobials cannot be used as empirical treatment. A limitation of the study was the fact that samples from other sites (e.g. rectum and pharynx) were not obtained in men who have sex with men.
When the antimicrobial sensitivity is unknown, current treatment guidelines recommend the use of extended spectrum cephalosporins (ceftriaxone or cefixime)11 and, recently, European guidelines recommend the use of dual therapy with ceftriaxone and azithromycin.12 However, decrease of susceptibility of N. gonorrhoeae to cephalosporins worldwide has also been described. In 2001, gonococci with reduced susceptibility to these antibiotics were first reported in Japan. Moreover, some of these reductions on susceptibility were accompanied with subsequent treatment failures.13 Highly ceftriaxone-resistant N. gonorrhoeae strains have been recently reported,14 and this fact shows that susceptibility to ceftriaxone is progressively decreasing. In Spain, in 2011 the first strain with high-level resistance to ceftriaxone was detected.15
In our health area, the majority of strains were susceptible to extended spectrum cephalosporins, but according to EUCAST three isolates were resistant to ceftriaxone and four strains were resistant to cefixime. On the other hand, 10 strains were resistant to azithromycin. In view of these results, the best empirical treatment with monotherapy for gonococcal infections in our health area should be the administration of ceftriaxone intramuscularly, although susceptibility study to antibiotics must be always performed in order to detect resistance to these antibiotics.
Recently, Serra-Pladevall et al.16 published the data of antimicrobial susceptibility of 100 NG strains isolated in Barcelona. In this study, three NG strains were nonsusceptible to ceftriaxone and ten were resistant to cefixime according to EUCAST criteria. The percentage of resistance to ceftriaxone was similar to the present study (4% vs 4.6%), although the percentage of resistance to cefixime was higher than that in our study (11% vs. 6.1%). However, if CLSI criteria were applied, the percentages of resistance to ceftriaxone and cefixime were low and similar in both studies.
Regarding susceptibility to antimicrobials, there are some differences in the breakpoint criteria between CLSI and EUCAST. According to CLSI, susceptibility to ceftriaxone and cefixime is defined when MIC is ≤0.25μg/mL, whereas strains with MIC >0.25μg/mL are defined as nonsusceptible. According to EUCAST, however, susceptibility to these antibiotics is defined when MIC is ≤0.12μg/mL, and those with MIC >0.12μg/mL are classified as resistant. On the other hand, only EUCAST has defined resistance to azithromycin (MIC >0.5μg/mL). In this sense and given these differences, there is a need to clarify the breakpoints for the study of susceptibility to these antimicrobials according to clinical and microbiological criteria.
With respect to the empirical treatment applied to our patients with resistance to ceftriaxone and/or cefixime, all patients were correctly treated with ceftriaxone (250mg, intramuscularly) and/or azithromycin 1g orally. Test of cure was only performed in one of these patients, but no failure in the treatment was clinically documented. However, it is strongly recommended to perform test of cure in all gonorrhoea cases in order to ensure eradication of infection and identify emerging resistance. Overall, test of cure was only carried out in 12 patients in this study, so efforts should be taken to warn the physicians about the importance of the follow-up to the patients.
Thirty-four different types were isolated in this study, although ST 1407 was the most frequent. This type was the most common in the recent report from Barcelona16 as well as in some European countries,17 being the wide dissemination of this type in Europe a recent phenomenon.18 It is necessary to perform typing of the isolates in order to detect the exact ST, because the detection of some types has been related with specific resistance phenotypes. Thus, ST 1407 is the clone that has been associated with resistance to ceftriaxone and cefixime4,19 and with treatment failures with extended spectrum cephalosporins.4 Our data also show the known evidence that all ST 1407 isolates in Europe are ciprofloxacin resistant, strongly associated with decreased susceptibility to cefixime.20 All our STs 1407 strains were resistant to ciprofloxacin, as well as all isolates published recently by Chisholm et al. coming from different countries of the European Union.20 This fact enhances the usefulness of molecular typing techniques as a method to predict antimicrobial resistance. Moreover, the results of the present study demonstrate that other types such as ST 9807, ST 7072 and ST 2992 showed decreased susceptibility to ceftriaxone and cefixime. To our knowledge, the strain ST 9807 that showed decreased susceptibility to cephalosporins has not been frequently reported and until now had not been linked with resistance to cephalosporins.
A recent report from the Centers for Disease Control and Prevention21 recommends the performance of nucleic acid amplification tests to detect gonorrhoea in all laboratories, but in our opinion, current strategies to control gonococcal infections should also include carrying microbiological cultures to all genital samples. Moreover, the introduction of correct empirical treatment based on antimicrobial susceptibility data at regional level and the adequate follow-up of patients in order to ensure the definitive eradication of the infection are the other cornerstones of infection control.
Due to the increase of N. gonorrhoeae multiresistant strains the development of antimicrobial surveillance programs is very important, not only at national or international level but also at regional level. The presence of quality regional surveillance data about antimicrobial resistance to N. gonorrhoeae is a good approach to estimate the global burden of resistance to this microorganism. The subsequent communication of data to physicians working in this health area is crucial to improve the control of gonococcal infections and to know the appropriate empirical treatment at each moment.
Conflict of interestThe authors declare no conflict of interest.
We would like to acknowledge Dr. Julio Vázquez from the National Centre of Microbiology (Instituto de Salud Carlos III, Madrid, Spain) for typing all N. gonorrhoeae strains.