Infections due to Enterococcus hirae have rarely been reported in humans but are not uncommon in mammals and birds. We describe a case of E. hirae bacteremia and pneumonia in a bird breeder and its potential relationship with regorafenib, a tirosin kinase inhibitor (TKI).
MethodsDescriptive study and review of the literature through a PubMed search of the cases described previously to date.
ResultsOnly seventeen cases have been described, mainly endocarditis, pyelonephritis, and intraabdominal infections. No cases of pneumonia have been reported so far. The recent increase in TKI use opens a new field to explore in infectious diseases due to both the exposure to these immunosuppressive drugs and the increased survival of subjects with severe underlying comorbidities.
ConclusionIn patients in contact with birds, immunosuppressed by their underlying morbidities and treated with regorafenib, clinicians should be aware of an increased risk of unusual potentially severe infections.
La infección por Enterococcus hirae se ha reportado raramente en humanos, pero no es infrecuente en mamíferos y aves. Describimos un caso de neumonía bacteriémica por Enterococcus hirae, y su posible relación con regorafenib, un inhibidor de la tirosina quinasa (TKI, por sus siglas en inglés).
MétodosEstudio descriptivo y revisión de la literatura mediante una búsqueda en PubMed de los casos descritos anteriormente hasta la fecha.
ResultadosSolo se han publicado 17 casos, principalmente endocarditis, pielonefritis e infecciones intraabdominales, sin ningún caso de neumonía. El reciente incremento en el uso de la TKI abre la puerta a un nuevo campo a explorar en enfermedades infecciosas debido tanto a la exposición a estos fármacos como al incremento en la supervivencia de individuos con importantes comorbilidades subyacentes.
ConclusiónEn pacientes en contacto cercano con aves, inmunodeprimidos por sus comorbilidades y en tratamiento con regorafenib, podría existir un mayor riesgo de infecciones inusuales potencialmente graves.
Enterococci are Gram-positive facultative anaerobes and are part of the normal gut microbiota. The main species causing infections in humans are Enterococcus faecalis and Eneterococccus faecium.1,2 In the last decade enterococci have been reported as the third most common cause of bacteraemia in humans.3
Enterococcus hirae is known to cause infections in psittacine birds but accounts for less than 1% of the enterococcal species isolated in human clinical samples.4
The first description of E. hirae infection in humans in 1998 reported a case of septicaemia in a patient with end stage renal disease in hemodialysis.5 We report a case of bacteraemic pneumonia caused by E. hirae in a subject receiving regorafenib. We discuss the potential relationship between regorafenib exposure and the development of this rare cause of infection.
Case reportA 57-year-old man with a history of type 2 diabetes mellitus, chronic obstructive pulmonary disease (COPD), hepatic cirrhosis Child Pugh B secondary to hepatitis C virus (HCV) and chronic alcoholism. He developed a hepatocarcinoma stage BCLC C. Initially, he was treated with radiofrequency ablation and alcoholization of multinodular hepatocellular carcinoma. Subsequently, due to intractable multifocal tumor progression, the second line of treatment was sorafenib, which was suspended after a few months due to intrahepatic progression. Finally, regorafenib was started with a stable clinical response. At admission, he had received 8 months of regorafenib.
The last hospital admission was the month before, due to a bilateral bronchopneumonia caused by Chlamydia psittaci. Detection of Legionella pneumophila and Streptococcus pneumoniae antigens in urine was negative, as well as sputum bacilloscopy, and bronchoalveolar lavage screening for bacteria, mycobacteria and fungi. The patient was a goldfinch breeder and some of them were apparently ill, with active wing molt, and he had isolated them in a separate room.
The patient reported dyspnea, disorientation and fever during 2 days. He had a temperature of 38°C, blood pressure 80/42mmHg, heart rate 73bpm, respiratory rate 32min−1; oxygen saturation at 95% on oxygen at 2L/min. There were no abnormal cardiac murmurs and no clinical signs of infective endocarditis and bronchospasm. In the neurological examination he had grade 2 encephalopathy.
The laboratory data demonstrated: white blood cells 13100/μL with left shift (neutrophils 82%), C-reactive protein of 31mg/L, lactic acid 3.33mmol/L, and bilirubin 3.65mg/dL. The arterial gases demonstrated pO2 64mmHg and pCO2 36mmHg with pH 7.46. Two sets of blood cultures obtained on admission yielded Enterococcus spp., which was identified at the species level as E. hirae by mass spectrometry (MALDI-TOF). The organism was susceptible to ampicillin, imipenem, gentamicin, ciprofloxacin, levofloxacin, vancomycin, teicoplanin, trimethoprim/sulfamethoxazole, linezolid and tigecyclin. Detection of L. pneumophila and S. pneumoniae antigens in urine was negative.
The chest X-ray showed a basal left condensation. Transthoracic echocardiogram study was normal, without vegetation images.
The patient was initially treated with intravenous piperacillin/tazobactam plus azithromycin and oral rifaximine. The treatment was subsequently de-escalated to amoxicillin-clavulanate for a total of 8 days. The infection was cured and control blood cultures were negative, with resolution of pneumonia.
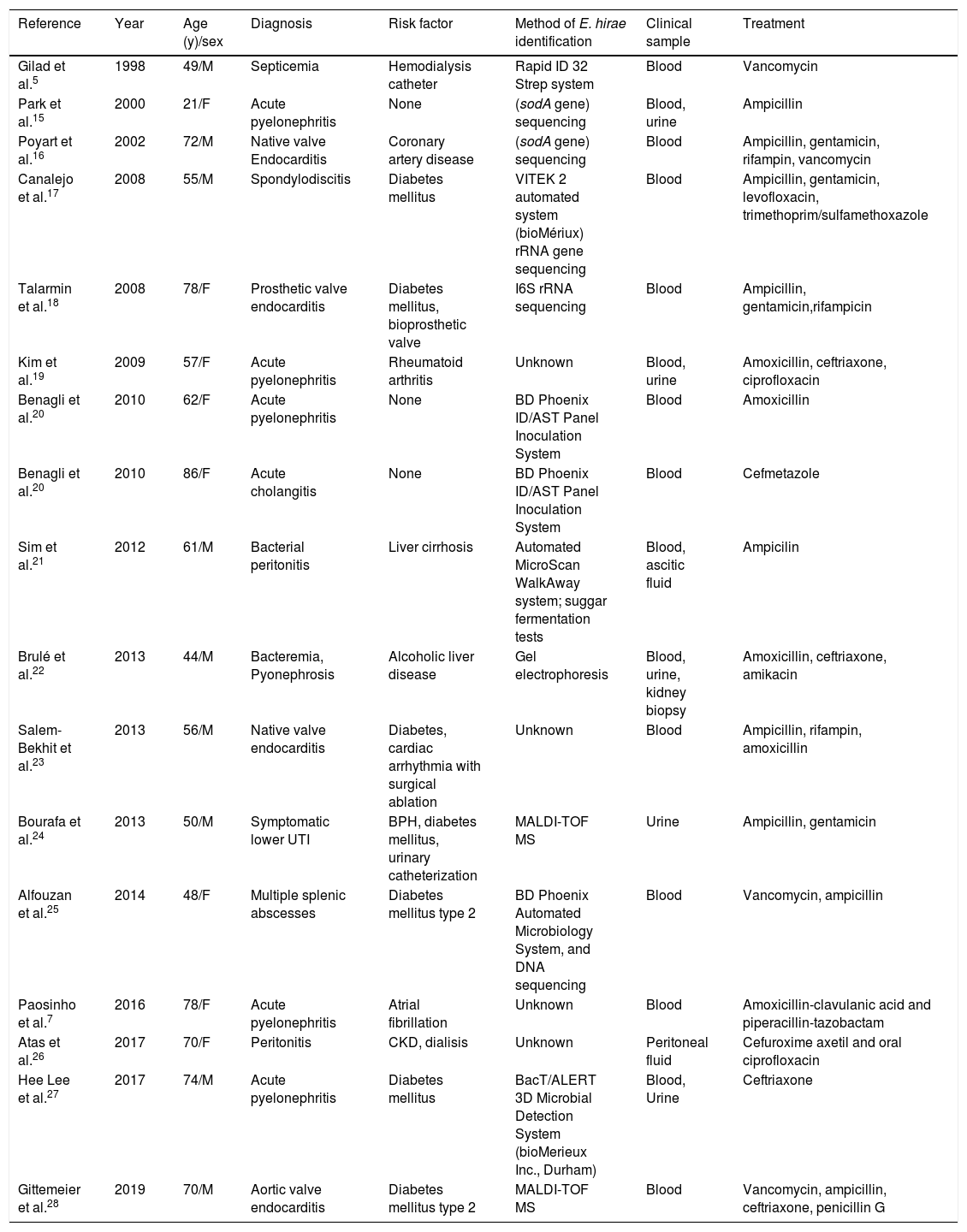
DiscussionE. hirae, originally described in 1985, is known to cause infections in various animal species, but is infrequent in humans.6 Only seventeen case reports of human infection due to this microorganism have been published to date (Table 1).7 All cases reported to date describe bacteremia, associated with different clinical conditions including endocarditis, pyelonephritis, and intraabdominal infections, which in some cases progressed to septic shock. To our knowledge, no cases of pneumonia have been described so far. In our patient, the episode of pneumonia could not be explained by the previous episode of C. psittaci three weeks before, which resolved completely both clinically and radiologicaly. In addition, in the chest X-ray we observed a new basal left condensation not previously observed.
Reported cases of human infections due to Enterococcus hirae. Updated from Paosinho et al.7
| Reference | Year | Age (y)/sex | Diagnosis | Risk factor | Method of E. hirae identification | Clinical sample | Treatment |
|---|---|---|---|---|---|---|---|
| Gilad et al.5 | 1998 | 49/M | Septicemia | Hemodialysis catheter | Rapid ID 32 Strep system | Blood | Vancomycin |
| Park et al.15 | 2000 | 21/F | Acute pyelonephritis | None | (sodA gene) sequencing | Blood, urine | Ampicillin |
| Poyart et al.16 | 2002 | 72/M | Native valve Endocarditis | Coronary artery disease | (sodA gene) sequencing | Blood | Ampicillin, gentamicin, rifampin, vancomycin |
| Canalejo et al.17 | 2008 | 55/M | Spondylodiscitis | Diabetes mellitus | VITEK 2 automated system (bioMériux) rRNA gene sequencing | Blood | Ampicillin, gentamicin, levofloxacin, trimethoprim/sulfamethoxazole |
| Talarmin et al.18 | 2008 | 78/F | Prosthetic valve endocarditis | Diabetes mellitus, bioprosthetic valve | I6S rRNA sequencing | Blood | Ampicillin, gentamicin,rifampicin |
| Kim et al.19 | 2009 | 57/F | Acute pyelonephritis | Rheumatoid arthritis | Unknown | Blood, urine | Amoxicillin, ceftriaxone, ciprofloxacin |
| Benagli et al.20 | 2010 | 62/F | Acute pyelonephritis | None | BD Phoenix ID/AST Panel Inoculation System | Blood | Amoxicillin |
| Benagli et al.20 | 2010 | 86/F | Acute cholangitis | None | BD Phoenix ID/AST Panel Inoculation System | Blood | Cefmetazole |
| Sim et al.21 | 2012 | 61/M | Bacterial peritonitis | Liver cirrhosis | Automated MicroScan WalkAway system; suggar fermentation tests | Blood, ascitic fluid | Ampicilin |
| Brulé et al.22 | 2013 | 44/M | Bacteremia, Pyonephrosis | Alcoholic liver disease | Gel electrophoresis | Blood, urine, kidney biopsy | Amoxicillin, ceftriaxone, amikacin |
| Salem-Bekhit et al.23 | 2013 | 56/M | Native valve endocarditis | Diabetes, cardiac arrhythmia with surgical ablation | Unknown | Blood | Ampicillin, rifampin, amoxicillin |
| Bourafa et al.24 | 2013 | 50/M | Symptomatic lower UTI | BPH, diabetes mellitus, urinary catheterization | MALDI-TOF MS | Urine | Ampicillin, gentamicin |
| Alfouzan et al.25 | 2014 | 48/F | Multiple splenic abscesses | Diabetes mellitus type 2 | BD Phoenix Automated Microbiology System, and DNA sequencing | Blood | Vancomycin, ampicillin |
| Paosinho et al.7 | 2016 | 78/F | Acute pyelonephritis | Atrial fibrillation | Unknown | Blood | Amoxicillin-clavulanic acid and piperacillin-tazobactam |
| Atas et al.26 | 2017 | 70/F | Peritonitis | CKD, dialisis | Unknown | Peritoneal fluid | Cefuroxime axetil and oral ciprofloxacin |
| Hee Lee et al.27 | 2017 | 74/M | Acute pyelonephritis | Diabetes mellitus | BacT/ALERT 3D Microbial Detection System (bioMerieux Inc., Durham) | Blood, Urine | Ceftriaxone |
| Gittemeier et al.28 | 2019 | 70/M | Aortic valve endocarditis | Diabetes mellitus type 2 | MALDI-TOF MS | Blood | Vancomycin, ampicillin, ceftriaxone, penicillin G |
BPH, benign prostatic hyperplasia; MALDI-TOF MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. CKD: chronic kidney diseases. UTI: urinary tract infection.
Although enterococci are not a usual cause of pneumonia, the findings on chest X ray with two positive blood cultures for E. hirae, as well as the negative results in all the microbiology work-up supported the diagnosis of E. hirae pneumonia.
In most cases reported, the source of E. hirae transmission to patients is unclear. Only one case reports an epidemiological contact with sick birds.7 Therefore, the mechanism of transmission to humans remains largely elusive. The majority of patients had immunosuppression such as diabetes mellitus, alcoholic liver disease or chronic renal failure.
In our case, there was a clear epidemiological relationship, which was the contact with sick birds, the animals most frequently affected by this pathogen. E. hirae can produce encephalomalacia with vascular thrombosis and meningitis in birds.8 The potential mechanism of transmission remains elusive, and both inhalation or direct skin contact could have a potential role. It is known that a prolonged hospital stay and a previous course of antibiotics can facilitate enterococcal infections. In this scenario, colonization by enterococci is a previous step for the development of the infection. We cannot rule out that our patient's skin was colonized by E. hirae from his sick birds.9
Enterococci are intrinsically resistant to different antibiotics such as cephalosporins and can acquire resistance to amoxicillin (through hiperproduction of PBP5) and aminoglycosides (through aminoglycoside-modifying enzymes). They also have a special ability to develop resistance to other antibiotics, either by acquisition of resistance genes located on plasmids or by spontaneous mutations. The ability of enterococci to survive in adverse conditions together with increasing numbers of patients with risk factors for an enterococcal infection (previous hospital admission, immunosuppression, previous use of antibiotics, etc.) has eventually increased the prevalence of infections due to these microorganisms. The acquired resistance to glycopeptides in enterococci can be attributed to the use of glycopeptide avoparcin in farms, the widespread use of vancomycin in clinical practice and the acquisition of resistance genes between different microorganisms.10
Agents targeting vascular endothelial growth factor (VEGF) – or specifically its tyrosine kinase domain (as well as those of other angiogenic signaling pathways) – have been developed in an attempt to improve antitumor efficacy and overcome resistance to VEGF blockade alone.11
Sorafenib, dasatinib, ruxolitinib, ibrutinib, tofazitinib, sunitinib, axitinib, and pazopanib are oral small-molecule tyrosine kinase inhibitors (TKIs) that have been associated with variable degrees of infectious complications, including fatal bacterial and fungal infections (particularly ibrutinib), with some contradictory results.12 Some of them even have a black box warning in their package insert information endorsed by drug regulatory agencies.
VEGF TKI seem to modulate the functionality of T cells. It is unlikely however, that such an effect exerts a negative impact on host immunity. In fact, in vivo studies suggest that sorafenib improves local tumor NK cells, T cells, macrophages and dendritic cell responses in murine models of hepatocarcinoma. However, it is unclear whether these properties act in the same direction outside the tumor environment, because some of them (sunitinib and sorafenib) have been found to inhibit the activation, proliferation and production of cytokines in peripheral T cells of the blood.13 These effects could potentially impact the response against some infections.
Likewise, therapy with sunitinib induced significant decreases in total leukocyte and neutrophil counts, as well as in certain peripheral blood lymphocyte subpopulations (total CD3+ and CD4+ subsets). These parameters return to baseline levels when sunitinib was discontinued.13
Recently, the European Medicines Agency (EMA) has approved regorafenib for the treatment of hepatocarcinoma, gastrointestinal stromal tumor, and metastatic colorectal carcinoma. This drug has potent receptor TKI activity targeted not only against VEGF but also against the angioprotein 1 receptor. Unlike previous VEGF TKI, regorafenib has not been associated with increased risk of infections in its pivotal phase III studies.14 However, it should be noted that clinical experience accumulated with regorafenib is more limited so far.
This recent increase in TKI use opens a new field to explore in infectious diseases, as the increased risk of infections can be associated as well with pre-existing immunosuppression due to cancer and various comorbidities (diabetes mellitus, cirrhosis) that together with the use of new molecules that inhibit immune signaling pathways, may increase the risk of presenting an infection.
In our case, we cannot confirm that E. hirae infection was only due to the treatment with regorafenib, because our patient had immune suppression induced by other multiple pathologies (type 2 diabetes, COPD, chronic alcoholism, HCV cirrhosis).
In conclusion, in patients taking care or in close contact with birds, immunosuppressed by their underlying morbidities and under treatment with regorafenib, clinicians should be aware of an increased risk for unusual potentially severe infections.
Post-marketing surveillance seems warranted with these new VEGF TKI, and increased awareness of infectious disease specialists will be pivotal.
Conflicts of interestThe authors declare no conflicts of interest.