Carbapenems are the beta-lactam antibiotics with the best spectrum of activity in the treatment of Pseudomonas aeruginosa infections. The objective of this study was to molecularly characterise a collection of carbapenem-resistant P. aeruginosa isolates (PARC).
MethodsA total of 85 PARC isolates were recovered from 60patients in the Hospital San Pedro, Logroño (period 2008–2011). Clonal relationship was determined using pulsed-field gel electrophoresis (PFGE), susceptibility testing to 15anti-pseudomonal agents was performed using the disk diffusion method, and alterations in oprD, characterisation of integrons and molecular typing (MLST) using PCR and sequencing.
ResultsThe 85 PARC were classified into 35 different PFGE profiles. Of the 61 selected strains from 60 patients all of them were multiresistant, although none of them showed a carbapenemase phenotype. High polymorphism was detected in OprD, emphasising that 59% of the strains had a premature stop codon. ISPa1328 and ISPsp4 insertion sequences truncated oprD gene into 2 strains (GenBank KF517097 and KF517098). Two-thirds (67%) of the strains showed class 1 integrons with genes encoding aminoglycoside modifying enzymes, and 2 of them carried a new integron: aac(3)-Ia+aadA1h, named In272, GenBank GQ144317. Four sequence types were detected (Strain Nos.): ST175 (35), ST308 (3), ST235 (2), and ST639 (1).
ConclusionMultidrug resistance, high polymorphism in oprD, a high percentage of integrons, moderate clonal relationship of strains, and the high epidemic dissemination of high-risk clones are clinical aspects of great concern in order to eradicate the spread of PARC.
Los carbapenémicos son los antibióticos betalactámicos con mayor espectro de actividad en el tratamiento de infecciones por Pseudomonas aeruginosa. El objetivo de este trabajo fue caracterizar molecularmente una colección de aislados de P. aeruginosa resistentes a carbapenémicos (PARC).
MétodosSe recogieron 85 aislados PARC de 60pacientes en el Hospital San Pedro, Logroño (período 2008-2011). La relación clonal se determinó por electroforesis en gel de campo pulsado (PFGE), la sensibilidad a 15antipseudomónicos por método de difusión en disco y las alteraciones en oprD, la caracterización de integrones y la tipificación molecular (MLST) por PCR y secuenciación.
ResultadosLas 85PARC se clasificaron en 35perfiles diferentes de PFGE. Se seleccionaron 61cepas de los 60pacientes y se observó que eran multirresistentes, aunque ninguna mostró fenotipo carbapenemasa. Se detectó un gran polimorfismo de OprD, destacando que el 59% de las cepas presentaban un codón de finalización prematuro. ISPa1328 e ISPsp4 truncaban el gen oprD en 2 cepas (GenBank KF517097 y KF517098). El 67% de las cepas presentó integrones de clase 1 con genes codificantes de enzimas modificantes de aminoglucósidos, 2 de las cuales portaban un nuevo integrón: aac(3)-Ia+aadA1h (nombrado In272, GenBank GQ144317). Se detectaron 4 secuencias tipo (ST) (número de cepas): ST175 (35), ST308 (3), ST235 (2) y ST639 (1).
ConclusiónLa multirresistencia, el alto polimorfismo de oprD, el alto porcentaje de integrones, la moderada relación clonal de las cepas y la elevada diseminación epidémica de clones de alto riesgo son aspectos de gran preocupación clínica para erradicar la diseminación de PARC.
The species Pseudomonas aeruginosa is one of the most clinically significant opportunistic and nosocomial pathogens. This is due to not only its high levels of adaptability, dissemination and persistence (mainly in moist environments), but also its intrinsic resistance to various microbial agents and its ease in acquiring new mechanisms of resistance. These things represent obstacles to successful treatment at hospitals when this bacterium is found to be involved in infectious processes. Furthermore, this pathogen causes serious infections in patients who suffer from chronic respiratory diseases and patients in a state of immunosuppression, mainly in a hospital environment, on intensive care units (ICUs) and on critical care haematology–oncology units.1,2
Carbapenems are the beta-lactam antibiotics with the broadest spectrum of activity. They are commonly used to treat infections caused by P. aeruginosa.3 To exert their effect, carbapenems must cross the cell wall. This occurs through outer membrane porins in Gram-negative bacteria. Specifically, OprD is a substrate-specific outer membrane porin for P. aeruginosa that enables the diffusion of carbapenems, basic amino acids and small peptides.3 However, in recent years, an increase in carbapenem-resistant P. aeruginosa (CRPA) strains has been observed.1,2 The main mechanisms of carbapenem resistance in P. aeruginosa are: 1) abnormalities in or loss of the OprD porin, 2) overexpression of active efflux pumps, 3) hyperproduction of chromosomal AmpC beta-lactamase and 4) production of carbapenemase enzymes. Class A carbapenemases, class B carbapenemases or metallo-beta-lactamases (MBLs) and class D carbapenemases or oxacillinases have been described.4 Although the prevalence of MBL-producing CRPA is increasing worldwide, intrinsic carbapenem resistance mechanisms such as the presence of abnormalities in OprD are the most common mechanisms.5–7
The objective of this study was to perform molecular characterisation of a set of CRPA isolates collected at Hospital San Pedro in Logroño, Spain. A phenotypic study of resistance to antipseudomonal agents was conducted, the mechanisms of carbapenem resistance involved were analysed, the presence of integrons was detected and molecular typing of the isolates selected was performed.
Material and methodsClinical isolatesA total of 85 P. aeruginosa isolates with resistance to at least one of the carbapenem antibiotics tested (imipenem, meropenem and/or doripenem), from clinical samples at Hospital San Pedro in Logroño, Spain, were collected from September 2008 to December 2011.
The isolates were obtained from different types of sample: wound (25), urine (16), ulcer (16), rectal swab (11), pharyngeal swab (6), sputum (3), conjunctival exudate (2) and other (6: inguinal swab, nasal exudate, aspirate, central catheter tip, blood culture and bronchoalveolar lavage). They were collected from 60 hospitalised patients (46 men and 14 women) with a mean age of 69 years (age range 16–99 years) primarily from the pulmonology (15%), surgery (15%), general medicine (8%), internal medicine (8%), haematology (7%), nephrology (5%), intensive care or intensive medicine (5%) and infectious disease (5%) departments.
Molecular typingThe clonal relationship between the isolates was studied through pulsed-field gel electrophoresis (PFGE). The bacterial DNA inserts were made in agarose according to the procedure described by Rojo-Bezares et al.8, and following digestion with the SpeI enzyme, the fragments were separated through PFGE under the following conditions: 2 pulsed linear ramps of 5–15s and 15–45s, respectively, for 10h each, while maintaining a voltage gradient of 6V/cm at 14°C. The band profiles obtained were analysed with the recommendations of Tenover et al.9 and then by the BioNumerics 2.0 software program (Applied Maths, Belgium).
The sequence type (ST) was determined through the multilocus sequence typing (MLST) technique, based on amplifying and sequencing 7 housekeeping genes and comparing these alleles with the database on http://pubmlst.org/paeruginosa/.
Studies of antibiotic sensitivitySensitivity to 15 antipseudomonal agents (ticarcillin, piperacillin, piperacillin–tazobactam [TZP], ceftazidime [CAZ], cefepime, aztreonam, imipenem [IPM], meropenem [MEM], doripenem [DOR], gentamicin, tobramycin, amikacin, ciprofloxacin, norfloxacin and colistin) was studied using the disk diffusion method, according to the recommendations of the Clinical and Laboratory Standards Institute (CLSI).10 The minimum inhibitory concentration (MIC) of the antibiotics IPM and MEM was determined through the agar dilution method,10 while the MIC of the antibiotics TZP and CAZ was determined through MIC test strips (Liofilchem, Italy; bioMérieux, France).
The extended-spectrum beta-lactamase (ESBL), metallo-beta-lactamase (MBL) and class A carbapenemase phenotypes were analysed using the double disk synergy test. The discs used in each case were as follows: 1) cefepime–amoxicillin/clavulanic acid–ceftazidime; 2) IPM–EDTA (0.5M, pH 8)–MEM, and 3) IPM–3-aminophenylboronic acid (300μg)–MEM. Hyperproduction of the AmpC beta-lactamase was investigated in CAZ-resistant strains using plates in the presence and in the absence of cloxacillin (250μg/ml) with CAZ E-test strips (bioMérieux, France). The strains PAO1 and PAOΔdB were used as negative and positive controls, respectively. The AmpC-hyperproducing isolate was identified when a difference of at least a double dilution between the MIC of CAZ and CAZ with cloxacillin was observed.
Analysis of genes determining metallo-beta-lactamases and class A carbapenemasesThe presence of genes encoding MBLs (IMP, VIM, GIM, SIM and SPM) or class A carbapenemases (KPC, SME, IMI and GES) was performed through multiple PCRs.8
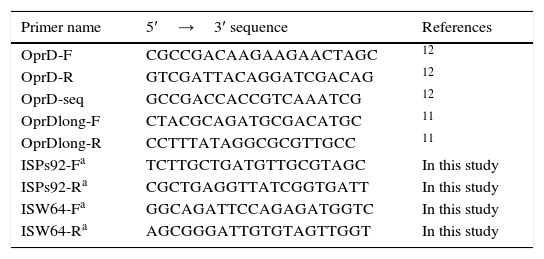
Characterisation of the OprD porinThe amino acid changes in the OprD protein were analysed using PCR,11,12 sequencing and subsequent comparison to the sequence of the P. aeruginosa strain PAO1 (access number in GenBank: AE004091) used as a reference strain. New sequencing primers were designed for the isolates Ps92 and W64 in which different insertion sequences truncated the oprD gene (Table 1).
Primers used in the analysis of the oprD gene.
| Primer name | 5′→3′ sequence | References |
|---|---|---|
| OprD-F | CGCCGACAAGAAGAACTAGC | 12 |
| OprD-R | GTCGATTACAGGATCGACAG | 12 |
| OprD-seq | GCCGACCACCGTCAAATCG | 12 |
| OprDlong-F | CTACGCAGATGCGACATGC | 11 |
| OprDlong-R | CCTTTATAGGCGCGTTGCC | 11 |
| ISPs92-Fa | TCTTGCTGATGTTGCGTAGC | In this study |
| ISPs92-Ra | CGCTGAGGTTATCGGTGATT | In this study |
| ISW64-Fa | GGCAGATTCCAGAGATGGTC | In this study |
| ISW64-Ra | AGCGGGATTGTGTAGTTGGT | In this study |
The detection of genes encoding type 1, 2 or 3 integrases as well as the presence of the 3′ conserved segment qacEΔ1+sul1 (characteristic of class 1 integrons) were studied through PCR. The polymorphism of the promoters Pc and P2 and the characterisation of the variable regions of the class 1 integrons were analysed using PCR and sequencing.13
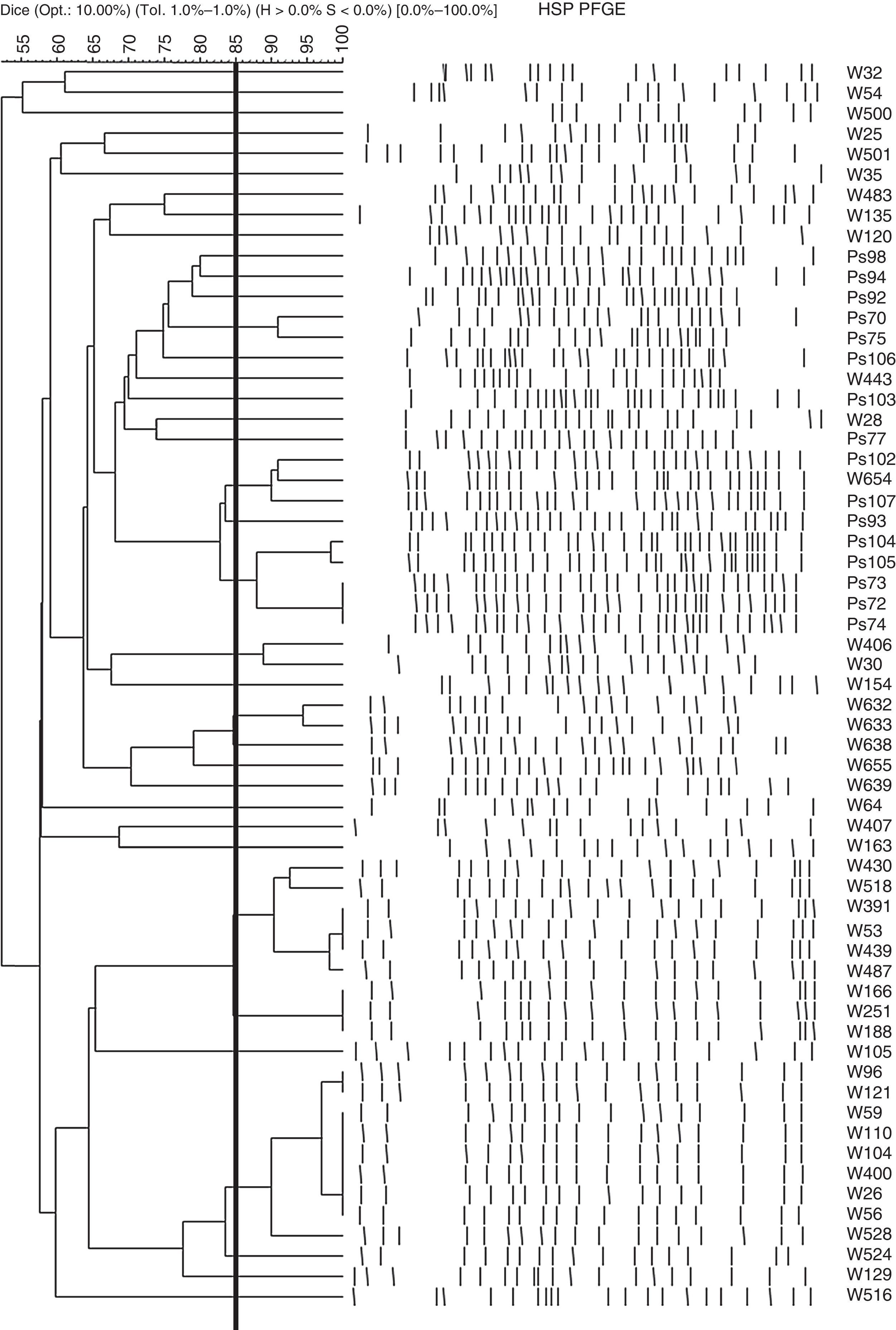
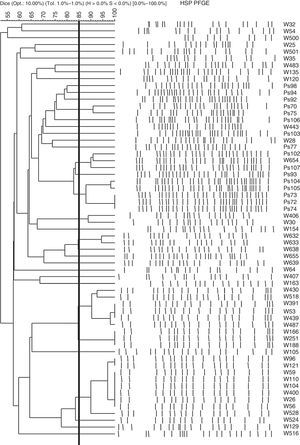
ResultsClonal relationship of the isolatesThe clonal relationship of the 85 CRPA strains isolated from 60 patients was studied through PFGE. Strains from a single patient were observed to show indistinguishable PFGE profiles, except one patient in whom the 2 strains isolated had different patterns. The selection criterion for continuing the study was the characterisation of one CRPA strain per patient (an exception was made to include the 2 strains with different PFGE patterns isolated in a single patient). Among the 61strains selected from the 60patients, 35different PFGE band patterns were observed. Fig. 1 shows the PFGE dendrogram obtained with these 61strains.
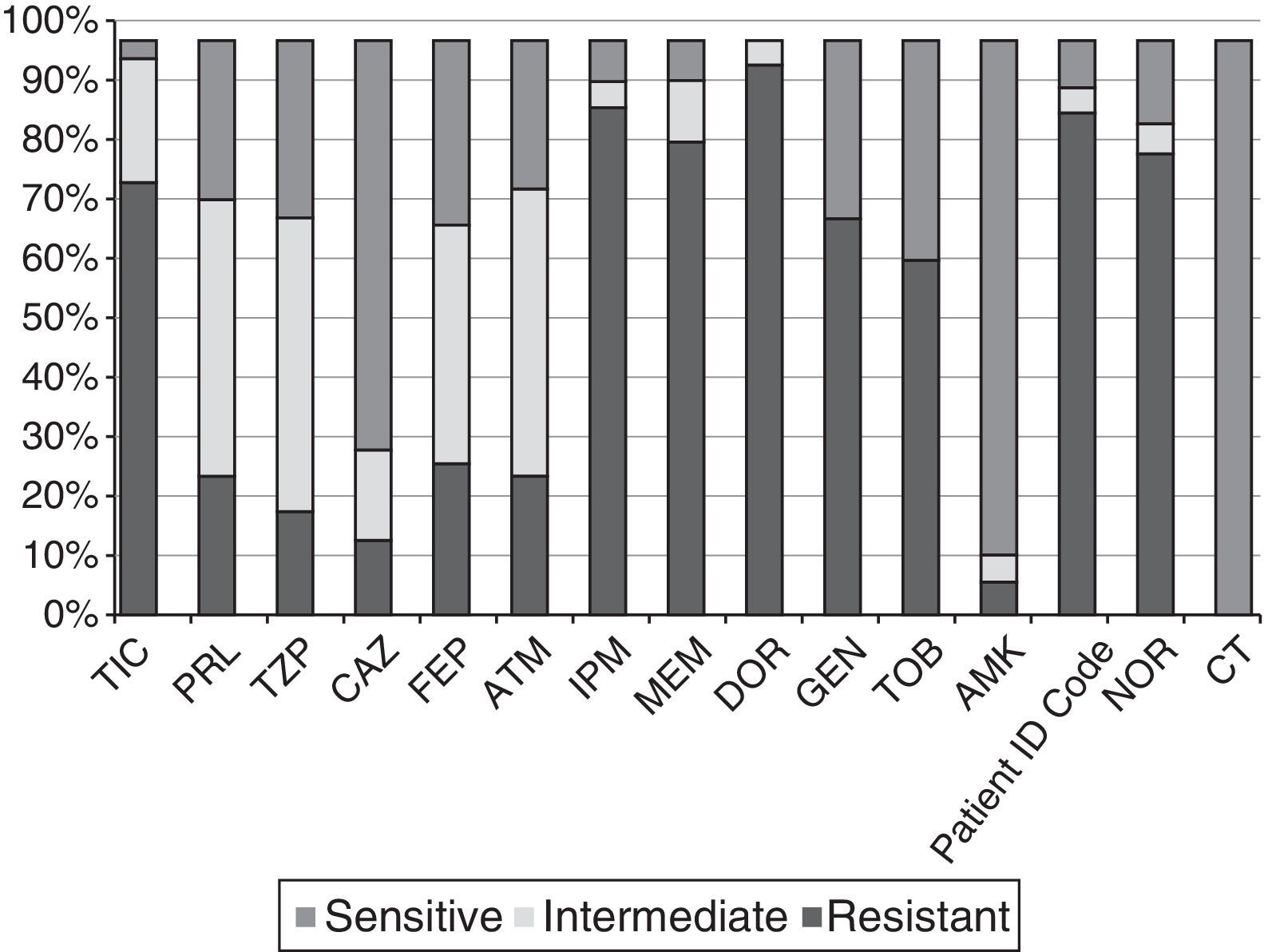
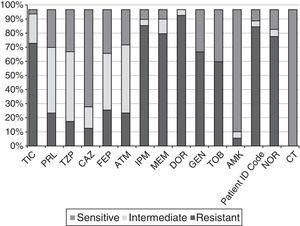
Profiles of antibiotic sensitivityFig. 2 shows the percentages of resistance to the 15 antibiotics tested in the 61 strains selected. All showed a multi-drug resistant phenotype according to the Magiorakos et al. criteria.14 The percentages of resistance to IPM, MEM, CAZ and TZP were 88%, 82%, 15% and 20%, respectively. The ranges of resistance observed according to MIC were 8–>64μg/ml for IMP; 8–128μg/ml for MEM; 32–>256μg/ml for CAZ, and >256μg/ml for TZP among the strains resistant to these antibiotics. None of the strains had the ESBL, MBL or class A carbapenemase phenotype, none was colistin-resistant, and only a single CAZ-resistant isolate (Ps75) showed an AmpC-hyperproducing phenotype. Coresistance of carbapenems (our selection criterion) with aminoglycosides and fluoroquinolones was observed.
Percentages of resistance of the 61 CRPA strains from HSP to the 15 antibiotics tested. AMK: amikacin; ATM: aztreonam; CAZ: ceftazidime; CIP: ciprofloxacin; CT: colistin; DOR: doripenem; FEP: cefepime; GEN: gentamicin; HSP: Hospital San Pedro; IPM: imipenem; MEM: meropenem; NOR: norfloxacin; PRL: piperacillin; TIC: ticarcillin; TZP: piperacillin–tazobactam; TOB: tobramycin.
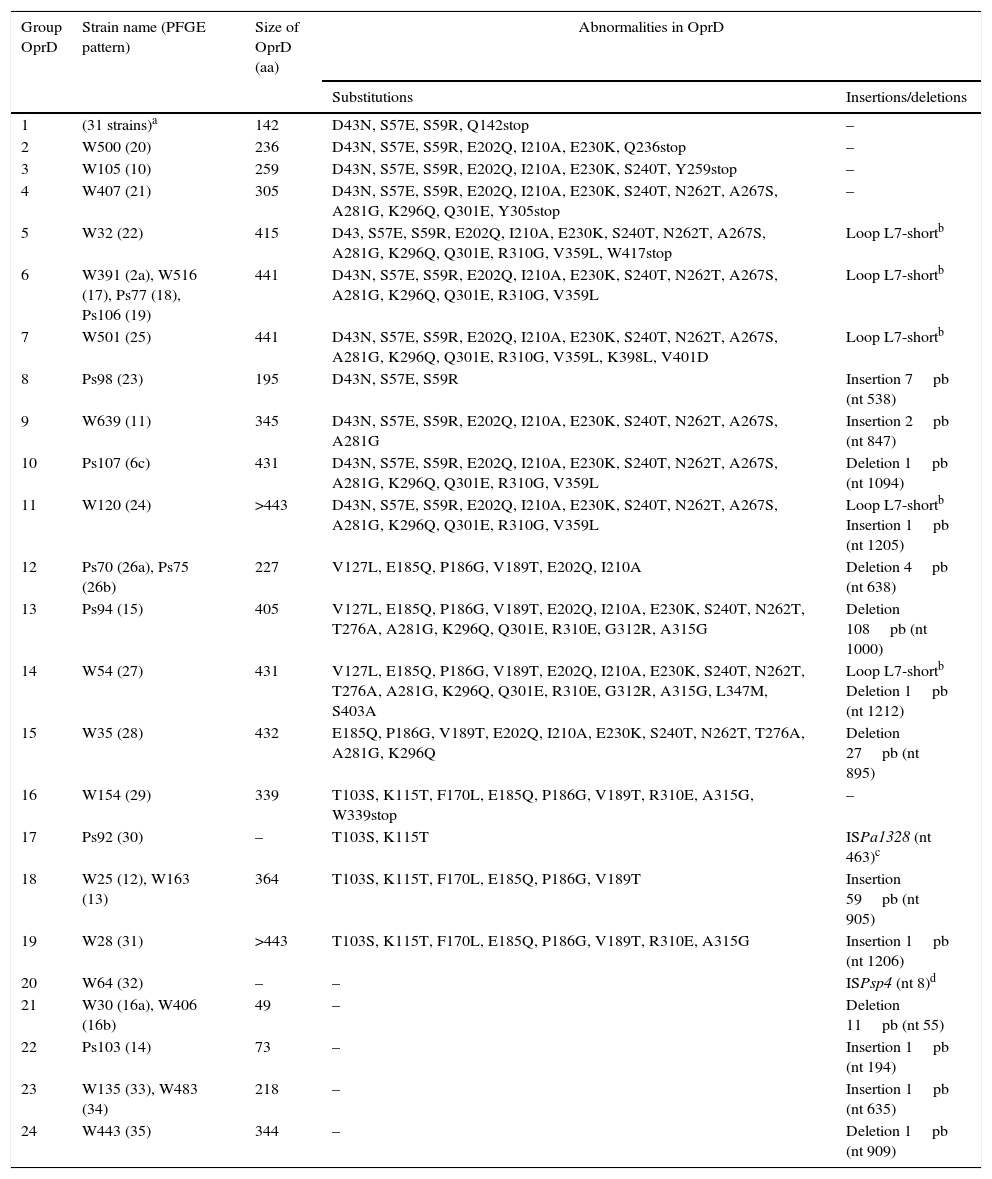
Table 2 shows the abnormalities in the OprD porin found in the 61CRPA strains selected. A wide variety of amino acid changes (substitutions, presence of insertions, deletions and premature termination codons) was detected in all of them. It should be noted that 59% (36/61) of the strains studied had a premature termination codon, and that 31 of these strains (belonging to 9 different PFGE patterns) showed it in amino acid 142 (Q142stop substitution).
Abnormalities found in the OprD porin of the 61 CRPA strains selected.
| Group OprD | Strain name (PFGE pattern) | Size of OprD (aa) | Abnormalities in OprD | |
|---|---|---|---|---|
| Substitutions | Insertions/deletions | |||
| 1 | (31 strains)a | 142 | D43N, S57E, S59R, Q142stop | – |
| 2 | W500 (20) | 236 | D43N, S57E, S59R, E202Q, I210A, E230K, Q236stop | – |
| 3 | W105 (10) | 259 | D43N, S57E, S59R, E202Q, I210A, E230K, S240T, Y259stop | – |
| 4 | W407 (21) | 305 | D43N, S57E, S59R, E202Q, I210A, E230K, S240T, N262T, A267S, A281G, K296Q, Q301E, Y305stop | – |
| 5 | W32 (22) | 415 | D43, S57E, S59R, E202Q, I210A, E230K, S240T, N262T, A267S, A281G, K296Q, Q301E, R310G, V359L, W417stop | Loop L7-shortb |
| 6 | W391 (2a), W516 (17), Ps77 (18), Ps106 (19) | 441 | D43N, S57E, S59R, E202Q, I210A, E230K, S240T, N262T, A267S, A281G, K296Q, Q301E, R310G, V359L | Loop L7-shortb |
| 7 | W501 (25) | 441 | D43N, S57E, S59R, E202Q, I210A, E230K, S240T, N262T, A267S, A281G, K296Q, Q301E, R310G, V359L, K398L, V401D | Loop L7-shortb |
| 8 | Ps98 (23) | 195 | D43N, S57E, S59R | Insertion 7pb (nt 538) |
| 9 | W639 (11) | 345 | D43N, S57E, S59R, E202Q, I210A, E230K, S240T, N262T, A267S, A281G | Insertion 2pb (nt 847) |
| 10 | Ps107 (6c) | 431 | D43N, S57E, S59R, E202Q, I210A, E230K, S240T, N262T, A267S, A281G, K296Q, Q301E, R310G, V359L | Deletion 1pb (nt 1094) |
| 11 | W120 (24) | >443 | D43N, S57E, S59R, E202Q, I210A, E230K, S240T, N262T, A267S, A281G, K296Q, Q301E, R310G, V359L | Loop L7-shortb Insertion 1pb (nt 1205) |
| 12 | Ps70 (26a), Ps75 (26b) | 227 | V127L, E185Q, P186G, V189T, E202Q, I210A | Deletion 4pb (nt 638) |
| 13 | Ps94 (15) | 405 | V127L, E185Q, P186G, V189T, E202Q, I210A, E230K, S240T, N262T, T276A, A281G, K296Q, Q301E, R310E, G312R, A315G | Deletion 108pb (nt 1000) |
| 14 | W54 (27) | 431 | V127L, E185Q, P186G, V189T, E202Q, I210A, E230K, S240T, N262T, T276A, A281G, K296Q, Q301E, R310E, G312R, A315G, L347M, S403A | Loop L7-shortb Deletion 1pb (nt 1212) |
| 15 | W35 (28) | 432 | E185Q, P186G, V189T, E202Q, I210A, E230K, S240T, N262T, T276A, A281G, K296Q | Deletion 27pb (nt 895) |
| 16 | W154 (29) | 339 | T103S, K115T, F170L, E185Q, P186G, V189T, R310E, A315G, W339stop | – |
| 17 | Ps92 (30) | – | T103S, K115T | ISPa1328 (nt 463)c |
| 18 | W25 (12), W163 (13) | 364 | T103S, K115T, F170L, E185Q, P186G, V189T | Insertion 59pb (nt 905) |
| 19 | W28 (31) | >443 | T103S, K115T, F170L, E185Q, P186G, V189T, R310E, A315G | Insertion 1pb (nt 1206) |
| 20 | W64 (32) | – | – | ISPsp4 (nt 8)d |
| 21 | W30 (16a), W406 (16b) | 49 | – | Deletion 11pb (nt 55) |
| 22 | Ps103 (14) | 73 | – | Insertion 1pb (nt 194) |
| 23 | W135 (33), W483 (34) | 218 | – | Insertion 1pb (nt 635) |
| 24 | W443 (35) | 344 | – | Deletion 1pb (nt 909) |
aa: amino acids; CRPA: carbapenem-resistant P. aeruginosa; nt: nucleotide; PFGE: pulsed-field gel electrophoresis.
Strains (PFGE pattern): W26(1a), W56(1a), W59(1a), W104(1a), W110(1a), W400(1a), W96(1b), W121(1b), W528(1c), W524(1d), W53(2a), W439(2a), W487(2b), W166(2c), W188(2c), W251(2c), W518(2d), W430(2e), Ps104(3a), Ps105(3b), W632(4a), W633(4b), W638(4c), Ps72(5), Ps73(5), Ps74(5), W654(6a), Ps102(6b), W129(7), W655(8), Ps93(9). All these strains belong to the clonal line ST175.
Similarly, different insertions/deletions were observed, in both size (1pb to >1000pb) and position (nucleotide 8 to nucleotide 1212). Notably, the strains Ps92 and W64 had the oprD gene interrupted by the insertion sequences ISPa1328 and ISPsp4, respectively. ISPa1328 belongs to the IS256 family and ISPsp4 belongs to the IS30 family. The sequences of the oprD gene in the strains Ps92 and W64 were new, and therefore they were entered in the GenBank gene database. Their access numbers were KF517097 and KF517098, respectively.
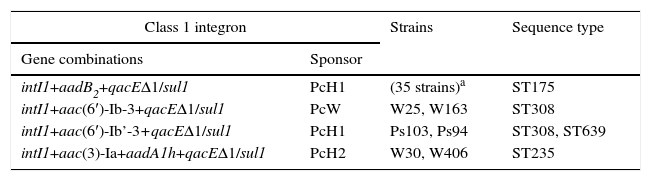
Characterisation of integrons and sequence typesA total of 67% (41/61) of the strains studied amplified the gene encoding integrase type 1 (intI1) and the 3′ conserved segment (qacEΔ1+sul1), while all of them were negative for integrases type 2 and 3. All intI1-positive strains included genes encoding aminoglycoside-modifying enzymes in their variable regions. It is important to note that none of our strains had 2 or more integrons simultaneously (Table 3).
Class 1 integrons and sequence types detected in the 41 intI1-positive strains.
| Class 1 integron | Strains | Sequence type | |
|---|---|---|---|
| Gene combinations | Sponsor | ||
| intI1+aadB2+qacEΔ1/sul1 | PcH1 | (35 strains)a | ST175 |
| intI1+aac(6′)-Ib-3+qacEΔ1/sul1 | PcW | W25, W163 | ST308 |
| intI1+aac(6′)-Ib’-3+qacEΔ1/sul1 | PcH1 | Ps103, Ps94 | ST308, ST639 |
| intI1+aac(3)-Ia+aadA1h+qacEΔ1/sul1 | PcH2 | W30, W406 | ST235 |
The most prevalent gene cassette was aadB2 regulated by a type 1 hybrid promoter (PcH1), present in 35 of the intI1-positive strains (85%). This gene cassette is identical to that described in the GenBank database with access number AJ871915; both have 4amino acid mutations compared to the gene cassette aadB (GenBank access number: L06418): K11Q, I60M, M69T and V142M.
In addition, other gene structures (number of strains) were found: aac(6′)-Ib-3 (2), aac(6′)-Ib’-3 (2) and the combination aac(3)-Ia+aadA1h (2). The difference between aac(6′)-Ib-3 and aac(6′)-Ib’-3 is a mutation in position 102 (L102S), while both genes had the N5T mutation with respect to the gene aac(6′)-Ib (GenBank access number: AF034958). The associated promoters in these cases were a weak promoter (PcW) for the gene cassette aac(6′)-Ib-3, while in the case of aac(6′)-Ib’-3, the promoter PcH1 was observed. Finally, the combination aac(3)-Ia+aadA1h was associated with a type 2 hybrid promoter (PcH2). This integron, first reported in this study and found in 2 strains (W30 and W406) from different patients and isolated in 2 different years (2008 and 2009, respectively), was deposited in the GenBank gene database with access number GQ144317 and was called In272 in the INTEGRALL database (http://integrall.bio.ua.pt/).
Moreover, the ST was analysed in all integron-carrying strains. Among the 41 strains, only 4 different STs were detected (number of strains): ST175 (35), ST308 (3), ST235 (2) and ST639 (1). Table 3 shows the association between the ST and the integron detected in the integron-carrying strains. All strains carrying the integron with the gene cassette aadB2 (11 different PFGE patterns) belonged to ST175. In addition, it should be noted that this group includes the 31 strains that had the Q142stop mutation in the OprD porin. For its part, ST308 was found to be associated with strains carrying different types of integrons-aac(6′)-Ib-3, aac(6′)-Ib’-3 and even one of those also related to ST639. ST308 and ST639 are not related, since the allelic combination of the two STs is completely different.
DiscussionP. aeruginosa is one of the most problematic pathogens in a clinical environment and the increase in carbapenem resistance in this species is very concerning worldwide due to the limitations that its clinical treatment involves. There are multiple publications on the presence of CRPA, including in the form of epidemic outbreaks, on the ICUs at different hospitals.15,16 However, in our study only 5% came from an intensive care or intensive medicine unit. Moreover, although the 61 CRPA strains selected showed a multi-drug resistant phenotype, none of them showed the ESBL, MBL or class A carbapenemase phenotype. In recent years, there have been manifold articles warning of the emerging problem of the spread of carbapenemase-carrying CRPA strains.7,17 However, the prevalence of MBL-producing CRPA strains in Spain is still relatively low compared to that in other countries such as Italy and Brazil.5–8,18 In fact, as observed in our study, the presence of abnormalities in OprD is the mechanism most commonly involved in imipenem resistance.6,16,19 Among the large number of abnormalities in OprD in the 61strains analysed, the presence of premature termination codons found in 36 of the strains studied or the truncation of oprD through inclusion of sequences or an insertion element inactivates OprD by linking it directly to imipenem resistance.8,12,19 The abnormality in OprD most commonly detected among our strains is Q142stop, which is associated with strains belonging to the clonal line ST175. This mutation in OprD has been found in multi-drug resistant strains from the clonal line ST175 that have spread at other Spanish hospitals.20 Two strains (Ps92 and W64) had the oprD gene truncated by the insertion sequences ISPa1328 and ISPsp4, respectively. Wolter et al.11 first reported the inactivation of the expression of the oprD gene through the presence of insertion sequences (ISPa1328 and ISPa1635), which would allow its strains an increase in carbapenem resistance. In recent years, there has been an increase in the number of studies reporting the inclusion of insertion sequences (ISPa27, ISPa45, ISPa46, ISPa47, ISPa133, ISPa1328, ISPa1635, ISPst12 and ISPpu21, among others) impeding the functionality of OprD. However, there is no specific position in which these insertion sequences interrupting the reading of the gene are inserted, nor is there any specific directionality or IS family to which they belong.8,12,19,21–23
Moreover, not all abnormalities found in our clinical strains are involved in the manifestation of resistance to imipenem and/or meropenem. There are amino acid changes (such as those observed in OprD patterns 6 and 7 in this study) that have been reported in both carbapenem-resistant and in carbapenem-sensitive strains.8,22,24 Similarly, the shortening of Loop-7 in 2 amino acids is an abnormality that does not directly involve carbapenem resistance because the porin continues to show a sufficient opening that does not interrupt the optimal penetration of the antibiotic.25 Other chromosomal mechanisms such as hyperproduction of active efflux pumps or of AmpC could be involved in carbapenem resistance.1,6
As in our study, in clinical strains of P. aeruginosa an association of resistance between beta-lactams, aminoglycosides and fluoroquinolones has been observed.2,26 This makes it difficult to select suitable antibiotic treatment to eradicate this species in the hospital environment.1,27 Regarding the values for resistance to other non-beta-lactam antibiotics, it should be noted that high aminoglycoside resistance (>60% for gentamicin and tobramycin) was found among our strains. The majority of aminoglycoside-resistant strains carried class 1 integrons, which in turn housed some gene encoding aminoglycoside-modifying enzymes in their variable region. Other studies have also reported that in P. aeruginosa the main gene cassettes in class 1 integrons confer resistance to beta-lactams and/or aminoglycosides.8,28
The variant of the gene cassette aadB (aadB2) was detected in 85% of integron-positive strains and all those attributed to the clone ST175. This association had already been reported by Nemec et al.29 in the Czech Republic and by prior studies by our research group.8 In recent years, the spread of high-risk clones in the P. aeruginosa species has played a fundamental role in the expansion of resistance worldwide.27 The sequence types ST235, ST111 and ST175 are the most common among the high-risk clones that have spread through hospitals worldwide. These sequence types are normally associated with multi-drug resistant, class 1 integron-carrying, carbapenem-resistant and/or MBL-producing strains.6,8,20,30,31
To sum up, carbapenemases were not detected among the CRPA strains isolated from clinical samples at the study hospital. However, the high polymorphism observed in the oprD gene, as well as the presence of insertion elements truncating it, would inactivate the OprD porin as they would be directly linked to carbapenem resistance among our strains. In addition, multi-drug resistant strains have been detected which, by housing class 1 integrons with gene cassettes involved in aminoglycoside resistance, belonged to high-risk epidemic clones (ST175 and ST235). The multi-drug resistant phenotype, high polymorphism of oprD, high percentage of integron-carrying strains, moderate clonal relationship of the strains and high epidemic spread of high-risk clones are matters of great concern requiring monitoring and follow-up that prevent the spread of these CRPA strains.
FundingThis study was funded in part by the Instituto de Salud Carlos III (project FIS PI12/01276). Vanesa Estepa received a predoctoral scholarship from Universidad de La Rioja (Spain) during the conduct of the experimental part of this study.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Estepa V, Rojo-Bezares B, Azcona-Gutiérrez JM, Olarte I, Torres C, Sáenz Y. Caracterización de mecanismos de resistencia a carbapenémicos en aislados clínicos de Pseudomonas aeruginosa en un hospital español. Enferm Infecc Microbiol Clin. 2017;35:141–147.
Part of this study was presented at the 14th Congress of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) (Abstract 720, Barcelona, Spain, 19–22 May 2010).