The prevalence of HIV-1 non-B variants is increasing in Spain, showing a higher number of transmitted drug resistance mutations (TDR) since 2002. This study presents the features of non-B-infected patients enrolled in the cohort of antiretroviral treatment (ART) naïve HIV-infected patients included in the Research Network on HIV/AIDS (CoRIS).
MethodsThe study includes a selected group of HIV-1 non-B-infected subjects from 670 subjects with pol sequences collected from 2004 to 2008 in the CoRIS cohort. Epidemiological-clinical-virological data were analyzed since cohort entry until October 2011, considering the presence or absence of treatment failure (TF).
ResultsEighty two non-B infected subjects with known HIV-1 variants were selected from 2004 to 2008 in the CoRIS cohort, being mainly female, immigrants, infected by recombinant viruses, and by heterosexual route. They had an intermediate TDR rate (9.4%), a high rate of TF (25.6%), of losses to follow-up (35%), of coinfections (32.9%), and baseline CD4+ counts ≥350cells/mm3 (61.8%). Non-B subjects with TF showed higher rates of heterosexual infection (85.7% vs. 69.5%, p<0.05), tuberculosis (30.8% vs. 9.1%, p=0.10) and hepatitis C (23.8% vs. 13.9%, p=0.34) coinfections and lower rates of syphilis (0% vs. 21.9%, p<0.05), and had more frequently received first-line ART including protease inhibitors (PIs) than patients without TF (70% vs. 30%, p<0.05). Interestingly, infection with non-B variants reduced the risk of TDR to nucleoside reverse transcriptase inhibitors and increased the risk to PIs.
ConclusionHIV-1 non-B-infected patients in Spain had a particular epidemiological and clinical profile that should be considered during their clinical management.
La prevalencia de variantes no-B del VIH-1 está aumentando en España, mostrando un incremento de las mutaciones de resistencia transmitidas (TDR) desde 2002. Este estudio muestra las características de los pacientes infectados por variantes no-B de la cohorte de infectados por VIH sin tratamiento antirretroviral de la Red de Investigación sobre VIH/SIDA (CoRIS).
MétodosDe 670 individuos en CoRIS con secuencias pol recogidas entre 2004 y 2008, se seleccionaron los pacientes infectados por variantes no-B. Se analizaron los datos epidemiológicos, clínicos y virológicos desde su inclusión hasta octubre de 2011, considerando la existencia de fracaso terapéutico (FT).
ResultadosLos 82 pacientes infectados por variantes no-B entre 2004 y 2008 fueron principalmente mujeres, inmigrantes, infectados por variantes recombinantes y transmisión heterosexual. Presentaron una tasa intermedia de TDR (9,4%) y elevada frecuencia de FT (25,6%), pérdidas de seguimiento (35%), coinfecciones (32,9%) y recuento basal de CD4+ ≥350células/mm3 (61,8%). Los pacientes no-B con FT vs. sin FT mostraron mayor porcentaje de transmisión heterosexual (85,7% vs. 69,5%, p<0,05), coinfecciones por tuberculosis (30,8% vs. 9,1%, p=0,10), hepatitis C (23,8% vs. 13,9%, p=0,34) y menores tasas de sífilis (0% vs. 21,9%, p<0,05). Además recibieron con mayor frecuencia tratamiento de primera línea con inhibidores de la proteasa (IP) (70% vs. 30%, p<0,05). La infección con variantes no-B redujo el riesgo de TDR a inhibidores de la transcriptasa inversa análogos de nucleósido y aumentó el riesgo a IP.
ConclusiónLos pacientes infectados por variantes no-B del VIH-1 en España presentan un perfil epidemiológico-clínico característico que deberá ser considerado durante su seguimiento.
Human immunodeficiency virus type 1 (HIV-1) shows a great genetic diversity and it is divided into four groups (M, N, O, P). HIV-1 Group M dominates the pandemic and is subdivided into 9 subtypes (A–D, F–H, J, K), at least 72 circulating recombinant forms (CRF) (http://www.hiv.lanl.gov/content/sequence/HIV/CRFs/CRFs.html) and multiple unique recombinant forms (URF). HIV-1 subtype B is the prevalent variant in high-income areas. However, subtypes other than subtype B and recombinants (HIV-1 non-B variants), responsible for 90% of the worldwide infections,1 are increasing in prevalence and heterogeneity in developed countries.2 In the Spanish cohort of antiretroviral (ARV)-naïve HIV-infected patients included in the Research Network on HIV/AIDS (CoRIS), the prevalence of these variants was estimated at 12.2%,3 being mainly CRFs3 and more common among foreigners.
The genetic particularities of non-B variants can potentially affect the HIV therapeutic management, including a faster rate of disease progression to AIDS,4 a different response and/or sensitivity to certain antiretroviral drugs due to genetic polymorphisms5 and a different rate of emergence of drug resistance mutations.6
In Spain, the patterns of transmitted drug resistance mutations (TDR) related to treatment failure (TF) during the antiretroviral treatment (ART) have been modified due to the increasing circulation of non-B variants among the HIV-1-infected population.7 Some studies have reported distinct temporal trends in TDR prevalence according to HIV-1 variants in different parts of Spain.8,9 On the other hand, non-B-infected patients are usually immigrants, which might be associated with obstacles for their clinical management such as cultural barriers and/or the presence of imported diseases.10 All these particularities make HIV-1 subtype characterization an important aspect to consider during the HIV-1 monitoring of ARV efficacy during the clinical management of infected individuals.
In the present study we have analyzed the clinical and epidemiological features in HIV-1 non-B-infected patients enrolled in the CoRIS cohort from 2004 to 2008. Furthermore, we have evaluated the rate of TF in this population, exploring the reasons for treatment change and the presence of drug resistance mutations.
Patients and methodsStudy populationCoRIS is an open, multicenter, prospective cohort of subjects over 13-years-old seen at 31 hospitals and one HIV diagnosis centre located throughout Spain since January 2004.11 Demographic, clinical and treatment-associated-information is periodically collected from the personal medical records during the patient's clinical follow-up. A total of 9667 subjects were enrolled in CoRIS between January 2004 and May 2013. About 65% of the antiretroviral-naïve patients started ART at entry.11 More than 30% of CoRIS subjects were immigrants.12 One of 10 HIV-1-infections in the CoRIS cohort was due to non-B variants, mainly recombinant viruses.3
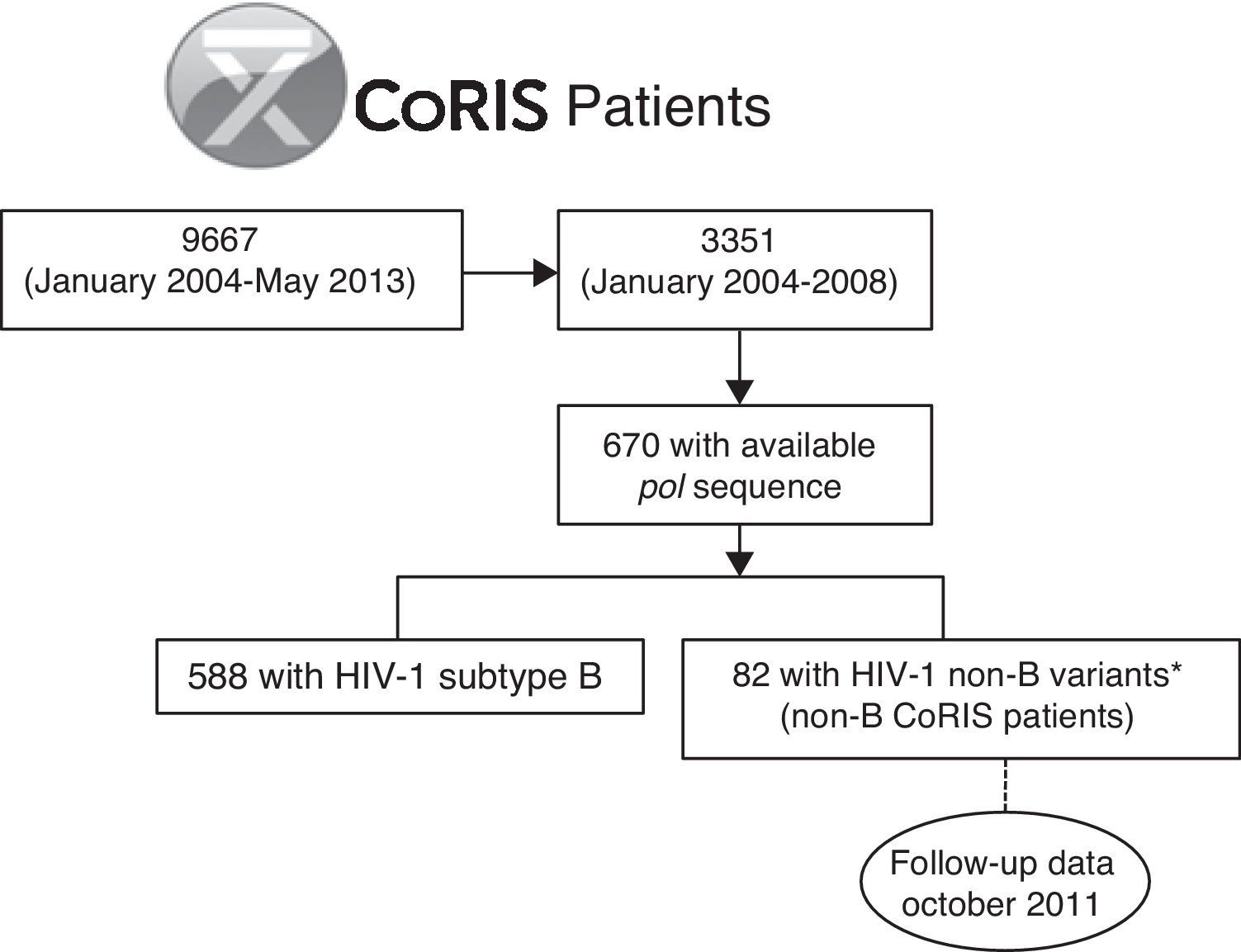
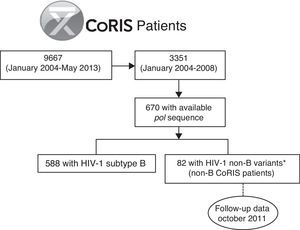
For the present study, we selected the 82 subjects from the CoRIS cohort previously identified as infected with HIV-1 non-B variants by phylogenetic analysis from the 670 patients with available pol sequence at enrollment among the 3351 subjects recruited from 2004 to 2008 in the cohort (non-B CoRIS patients),3 with follow up clinical data collected until October 2011 (Fig. 1). Not all pol sequences were available in that period since resistance testing in naïve patients was not recommended in the Spanish guidelines until early 2007.13 Longitudinal pol non-B sequences collected during clinical follow-up of these 82 non-B patients were recovered from the CoRIS database when available. Among HIV-1 non-B variants, non-B subtypes and recombinants (CRFs and URFs) were previously characterized by phylogenetic analysis of pol sequences, the gold standard method for HIV-1 variant characterization, and bootscanning analysis for complex recombinants.3 This study was approved by a review board and the Ethical Committee of the CoRIS cohort. It was designed to protect the rights of all subjects involved under the appropriate local regulations.
Analyzed variablesThe clinical and epidemiological features were collected in October 2011 from the 82 HIV-1 non-B infected patients identified in CoRIS until 2008. Besides the HIV-1 variant at pol, the studied variables were sociodemographic (age, gender, country of origin, educational level), clinical (HIV transmission route, date of enrollment, CDC category, ART, TF experience, losses to follow-up, time of clinical follow-up and visit frequency), immunological (CD4 counts) and virological (HIV-1 viral load or VL, TDR presence at cohort entry, coinfections with syphilis, tuberculosis, virus hepatitis C (HCV) and hepatitis B virus (HBV). Data were collected at CoRIS cohort entry and during the clinical follow-up until October 2011. TDR was evaluated in available pol sequences using the calibrated population resistance tool (http://cpr.stanford.edu/cpr.cgi) and interpreted following the surveillance drug resistance mutations list update in 2009.14 ART was classified according to the national guidelines (Spanish AIDS Study Group-National Plan for AIDS-GeSIDA-PNS-Guidelines).15 Losses to follow-up were considered in all subjects without available data during the last year of follow-up and without evidence of their death.
Treatment failure definitionTF was defined following WHO specifications (http://www.who.int/hiv/pub/guidelines/arv2013/art/arv2013_chapter07_low.pdf?ua=1) and two groups were defined with different criteria. The first one included patients with a persistently detectable plasmatic VL higher than 50 HIV-1-RNA copies/ml (c/ml) after at least 24 weeks of ARV exposure in at least two consecutive viraemia quantifications within three months, with adherence support between measurements. The second group included subjects with two consecutive detectable VL determinations after reaching undetectable VL (≤50c/ml).
Data analysisStatistical analyses were performed using Epi Info v6.0 (Centers for Disease Control and Prevention, Atlanta, GA, USA). Significance was set at p<0.05. The clinical follow-up time and visits data from the patients under study were presented as mean values with their 25th and 75th percentiles. Descriptive analyses for studied variables were presented using absolute numbers and percentages.
ResultsClinical and epidemiological features of non-B CoRIS patientsThe 82 non-B CoRIS patients were recruited from January 2004 to October 2008 (36% of them in 2007) and were under follow-up in 15 hospitals and one HIV diagnosis centre from 7 Spanish Autonomous Regions from Spain, mainly (47.5%) in Madrid. They were mainly infected 59 cases, 71.9%) by HIV-1 recombinant viruses at pol (58.5% CRF and 13.4% URF), the most being representative CRF02_AG (37.8%), followed by CRF14_BG (4.9%), CRF01_AE, CRF03_AB, CRF19_cpx (2.4% each) and CRF06_cpx, CRF11_cpx, CRF12_BF, CRF15_01B, CRF20_BG, CRF28_BF and CRF42_BF (1.2% each). Among “pure” subtypes, sequences from sub subtype A1 were the most represented (11%), followed by F1 and F2 (4.9% each), C (3.7%), G (2.4%) and D (1.2%), as previously reported.3
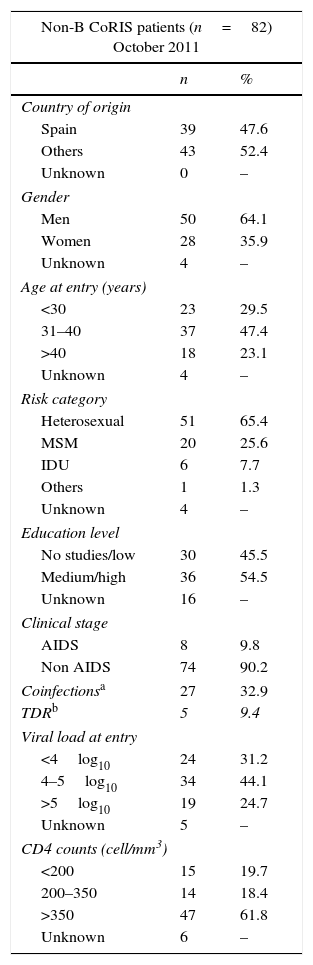
Table 1 shows the clinical and epidemiological features recorded in 2011 in these 82 CoRIS patients infected with non-B subtypes or recombinants. The mean age of non-B CoRIS subjects at cohort enrollment was 36 years (21–67 range), only 6% were older than 50 years and 12% were younger than 25 years. Most of them were men (64.1%) and foreigners (52.4%) with different origins: 12 Central and South Americans, 13 Sub-Saharan Africans, 8 East Europeans or Russians, 9 North Africans and 1 Asian. The main HIV transmission route was by sexual intercourse (91%). Among those 66 with available information on educational level at CoRIS enrollment, 12.1% had not finished primary school, a third had completed primary (33.3%) or secondary (36.4%) education and 18.2% reached university degree. The majority (90.2%) did not present AIDS symptoms. Up to half (61.8%) of patients had high CD4 counts (>350cells/mm3) with a mean CD4+ cell count of 433cells/mm3 (15–1168 range) and most (68.8%) had a mean VL of 4log10 (mean 268,788c/ml).
Clinical and epidemiological features of 82 HIV-1 non-B CoRIS patients.
| Non-B CoRIS patients (n=82) October 2011 | ||
|---|---|---|
| n | % | |
| Country of origin | ||
| Spain | 39 | 47.6 |
| Others | 43 | 52.4 |
| Unknown | 0 | – |
| Gender | ||
| Men | 50 | 64.1 |
| Women | 28 | 35.9 |
| Unknown | 4 | – |
| Age at entry (years) | ||
| <30 | 23 | 29.5 |
| 31–40 | 37 | 47.4 |
| >40 | 18 | 23.1 |
| Unknown | 4 | – |
| Risk category | ||
| Heterosexual | 51 | 65.4 |
| MSM | 20 | 25.6 |
| IDU | 6 | 7.7 |
| Others | 1 | 1.3 |
| Unknown | 4 | – |
| Education level | ||
| No studies/low | 30 | 45.5 |
| Medium/high | 36 | 54.5 |
| Unknown | 16 | – |
| Clinical stage | ||
| AIDS | 8 | 9.8 |
| Non AIDS | 74 | 90.2 |
| Coinfectionsa | 27 | 32.9 |
| TDRb | 5 | 9.4 |
| Viral load at entry | ||
| <4log10 | 24 | 31.2 |
| 4–5log10 | 34 | 44.1 |
| >5log10 | 19 | 24.7 |
| Unknown | 5 | – |
| CD4 counts (cell/mm3) | ||
| <200 | 15 | 19.7 |
| 200–350 | 14 | 18.4 |
| >350 | 47 | 61.8 |
| Unknown | 6 | – |
IDU, injection drug users; MSM, men who have sex with other men. Percentages pondered only from patients with available data.
Nearly a third (32.9%, 27 subjects) of the 82 non-B CoRIS HIV-1 cohort with available serological information were coinfected with other infectious agents (Tables 1 and 2). Among these 27 non-B patients, in 10 (15.5%) cases were coinfected with syphilis, 8 (18.2%) with tuberculosis, 12 (16%) with HCV and 2 (2.9%) with HBV (Table 2).
Differences in clinical-epidemiological features of non-B patients according to TF.
| Features (cases with available information) | With TF (n=21) | Without TF (n=38) | p-Value | Unknowna (n=23) | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Country of origin (n=82) | |||||||
| Spain | 9 | 42.9 | 19 | 50 | NS | 11 | 47.8 |
| Sub-Saharan Africa | 6 | 28.6 | 5 | 13.2 | NS | 2 | 8.7 |
| North Africa | 3 | 14.3 | 4 | 10.5 | NS | 2 | 8.7 |
| Eastern Europe and Russia | 3 | 14.3 | 1 | 2.6 | 0.09 | 4 | 17.4 |
| Latin America | 0 | – | 9 | 23.7 | – | 3 | 13 |
| Asia | 0 | – | 0 | – | – | 1 | 4.3 |
| Gender (n=78) | |||||||
| Men | 12 | 57.1 | 27 | 71.1 | NS | 11 | 57.9 |
| Women | 9 | 42.9 | 11 | 28.9 | NS | 8 | 42.1 |
| Age at entry (n=78) | |||||||
| <30 years | 7 | 33.3 | 8 | 21.1 | NS | 8 | 42.1 |
| 31–40 years | 10 | 47.6 | 17 | 44.7 | NS | 10 | 52.6 |
| >40 years | 4 | 19.1 | 13 | 34.2 | NS | 1 | 5.3 |
| Risk category (n=78) | |||||||
| Heterosexualb | 18 | 85.7 | 23 | 60.5 | 0.04 | 10 | 52.6 |
| IDU | 3 | 14.3 | 1 | 2.6 | 0.09 | 2 | 10.5 |
| MSM | 0 | – | 13 | 34.2 | – | 7 | 36.8 |
| Others | 0 | – | 1 | 2.6 | – | 0 | – |
| Educational level (n=66) | |||||||
| No studies | 4 | 22.2 | 3 | 10 | NS | 1 | 5.6 |
| Low | 4 | 22.2 | 12 | 40 | NS | 6 | 33.3 |
| Medium | 7 | 38.9 | 9 | 30 | NS | 8 | 44.4 |
| High | 3 | 16.7 | 6 | 20 | NS | 3 | 16.7 |
| Clinical stage (n=76) | |||||||
| A. Asymptomatic or primoinfection | 19 | 90.5 | 27 | 73 | NS | 16 | 88.9 |
| B. Non-AIDS symptoms | 2 | 9.5 | 3 | 8.1 | NS | 1 | 5.6 |
| C. AIDS | 0 | – | 7 | 18.9 | – | 1 | 5.6 |
| Presence of coinfectionc | 8 | 38.1 | 12 | 31.6 | 7 | 30.4 | |
| Type of coinfection | |||||||
| Syphilis | 0 | 0 | 7 | 21.9 | 0.03 | 3 | 18.8 |
| Tuberculosis | 4 | 30.8 | 2 | 9.1 | NS | 2 | 22.2 |
| HCV | 5 | 23.8 | 5 | 13.9 | NS | 2 | 11.1 |
| HBV | 0 | – | 1 | 2.9 | NS | 1 | 7.1 |
| Longitudinal sequences number | |||||||
| One | 13 | 61.9 | 31 | 81.6 | NS | 23 | 100 |
| Two | 6 | 28.6 | 5 | 13.2 | NS | 0 | – |
| Three | 2 | 9.5 | 2 | 5.3 | NS | 0 | – |
| Viral load at entry (n=77)d | |||||||
| >5log10 | 3 | 14.3 | 12 | 31.6 | NS | 3 | 16.7 |
| 5–4log10 | 11 | 52.4 | 15 | 39.5 | NS | 9 | 50 |
| <4log10 | 7 | 33.3 | 11 | 28.9 | NS | 6 | 33.3 |
| CD4 counts at entry (n=76) | |||||||
| <200cell/mm3 | 5 | 25 | 9 | 23.7 | NS | 1 | 5.6 |
| 200–350cell/mm3 | 5 | 25 | 7 | 18.4 | NS | 2 | 11.1 |
| >350cell/mm3 | 10 | 50 | 22 | 57.9 | NS | 15 | 83.3 |
| HIV-1 variante | |||||||
| Subtypes | 6 | 28.6 | 10 | 26.3 | NS | 7 | 30.4 |
| non CRF02_AG recombinants | 6 | 28.6 | 13 | 34.2 | NS | 10 | 43.5 |
| CRF02_AG | 9 | 42.9 | 15 | 39.5 | NS | 6 | 26.1 |
| TDR | 1 | 0.02 | 3 | 0.06 | NS | 1 | 0.02 |
Available data in October 2011.
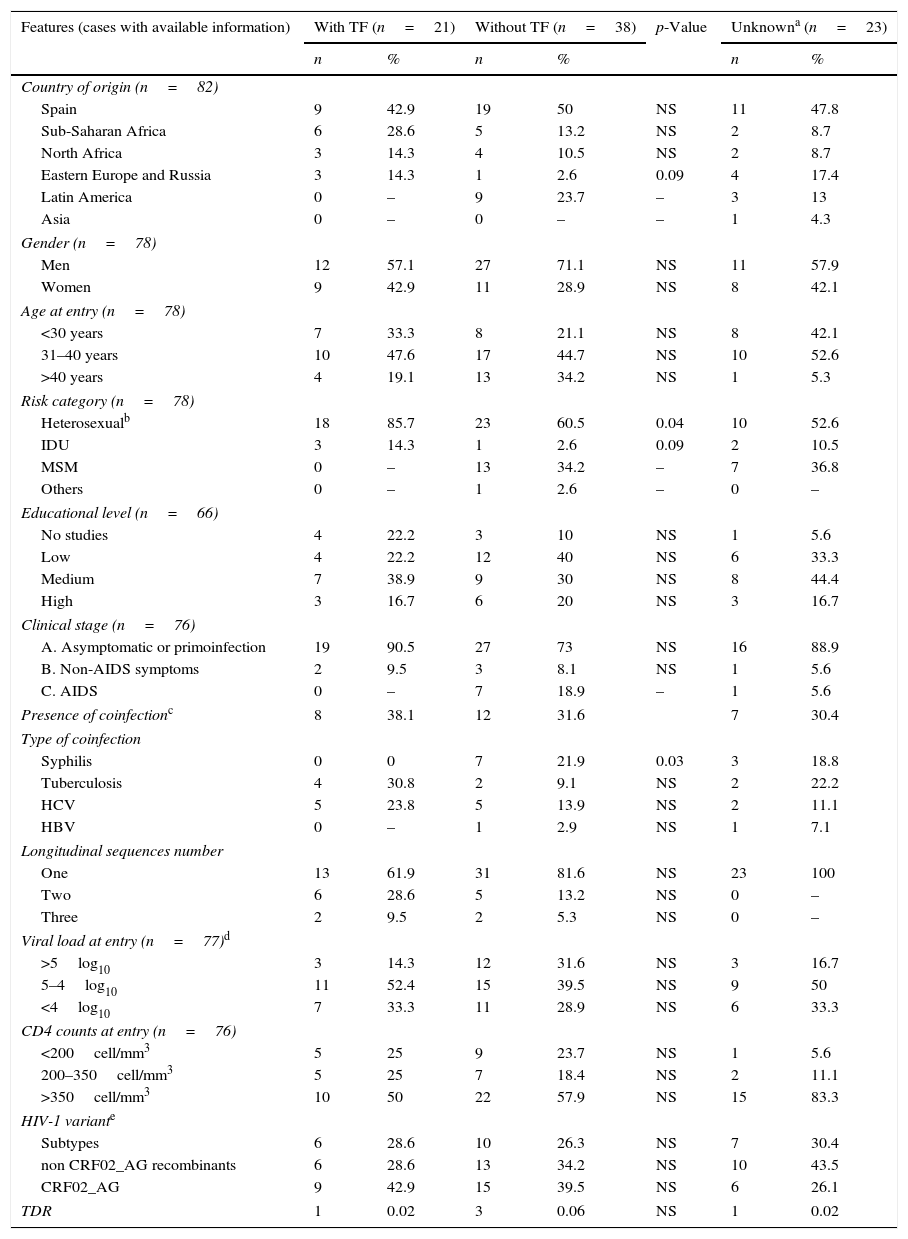
Serological information of other coinfections were available in 66 (80.5%) subjects for syphilis, in 44 (53.7%) for tuberculosis, in 75 (91.5%) for HCV and in 70 (85.4%) for HBV.
HIV-1 variant distribution among the 82 non-B patients according to TF (with/without/unknown) is: A1 (3/4/2), C (1/1/1), D (1/0/0), F1 (0/2/2), F2 (0/2/2) and G (1/1/0) for subtypes and CRF01_AE (0/0/2), CRF02_AG (9/15/6), CRF03_AB (0/2/0), CRF06_cpx (0/0/1), CRF11_cpx (1/0/0), CRF12_BF (0/1/0), CRF14_BG (2/0/2), CRF15_01B (0/1/0), CRF19_cpx (0/2/0), CRF20_BG (0/1/0), CRF28_BF (0/1/0), CRF42_BF (1/0/0) and URF (2/5/5) for recombinants. TF, treatment failure; IDU, injection drug users; MSM, men who have sex with other men; HCV, hepatitis C virus; HBV, hepatitis B virus; TDR, drug resistance mutations; NS, not significant difference or p>0.05. Percentages pondered only from patients with available data.
Table 3 summarizes the mean values of clinical follow-up period and visit frequency for the total CoRIS cohort and the study non-B CoRIS group. These last presented a mean of nearly 4 years of follow-up, a mean of 11 visits and mean time between visits of 3.8 months. Although no deaths occurred until October 2011 in the non-B group, nearly a third (35%) were losses to follow-up in the corresponding hospital, which was more frequent in the middle-aged patients, in the non-Spanish population, in subjects with medium educational level and in those with a CD4 count over 350cells/mm3 and 4–5log10c/ml of VL at the time of recruitment (data not shown).
Clinical follow-up among non-B group and total CoRIS cohort.
| Years under follow up (perc.) | Number of visits (perc.) | Days among visitsb (perc.) | |
|---|---|---|---|
| CoRISa(n=9667) | 3 (1–5.2) | 9 (4–15) | 122 (97–155) |
| Non-B CoRIS group | |||
| Total (n=82) | 3.8 (3–5.3) | 11 (4–16) | 115 (104–130) |
| With TF (n=21) | 4 (3–5.8) | 13 (5–19) | 128 (107–139) |
| Without TF (n=38) | 3.9 (3.1–4.9) | 13 (10–17) | 119 (108–132) |
| Unknown (n=23) | 3.1 (1.4–4.3) | 5 (1–8) | 140 (103–151) |
We distributed the non-B CoRIS patients into three groups, according to the TF experience during the follow-up (Table 2). Among the 82 non-B subjects, 21 (25.6%) had presented TF, 38 (46.3%) never had TF and had reached and maintained undetectable viraemia during the follow-up and 23 (28.1%) did not have enough information related to ART experience or lacked VL data. Thus, the TF rate could be underestimated in the non-B CoRIS cohort, since it would have risen from 25.6% to 35.6% if only those with available information were considered. From the 21 patients with TF, 10 (47.6%) presented persistent detectable HIV-1 VL, with mean VL values at entry of 95,934c/ml, persistently detectable after at least 24 weeks on ART. The remaining 11 (52.4%) non-B CoRIS patients with TF presented at least two consecutive detectable VL determinations after reaching undetectable VL (≤50c/ml), with a mean VL of 36,214c/ml at entry. Among them, 2 subjects presented two VL rebounds and 1 patient three.
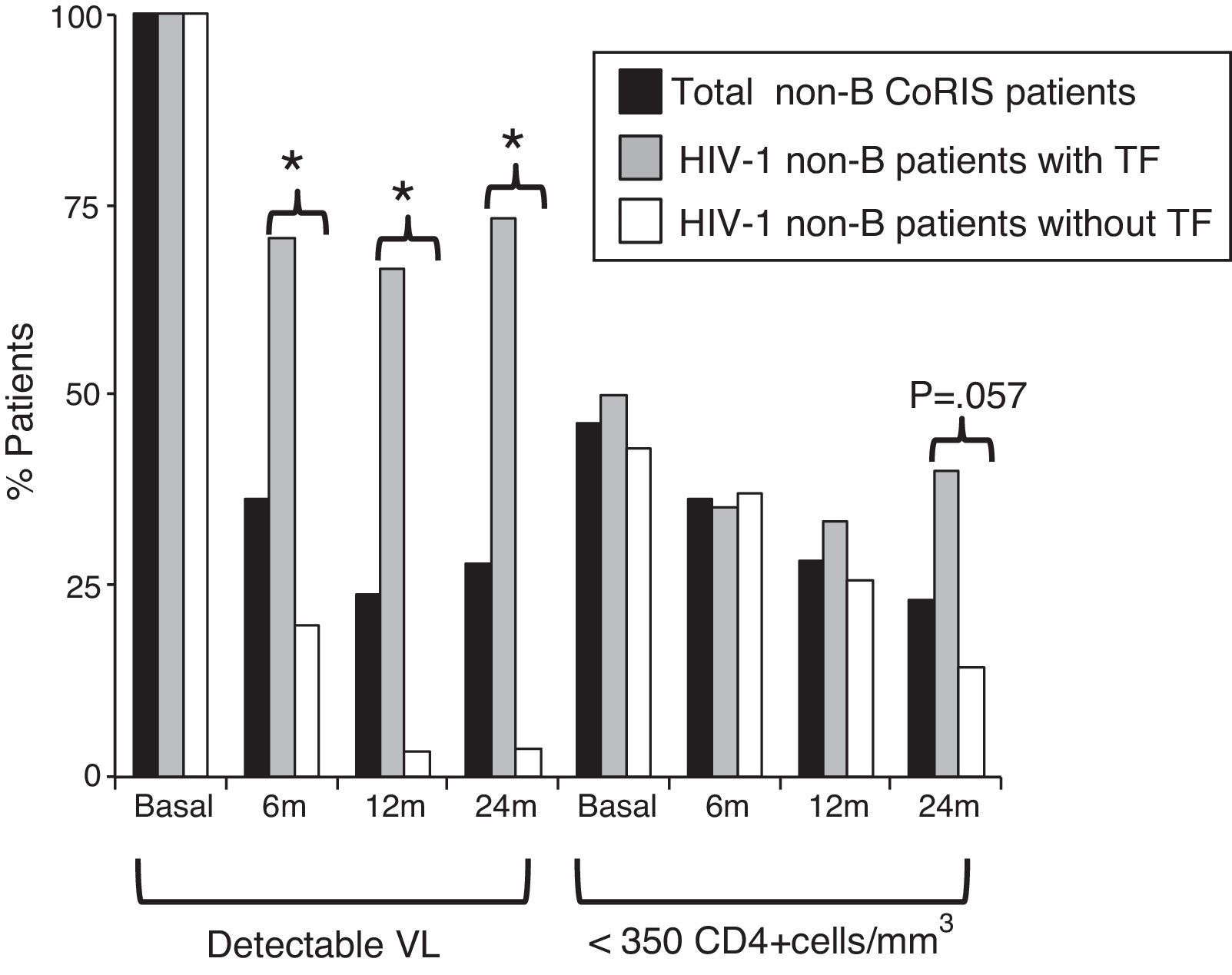
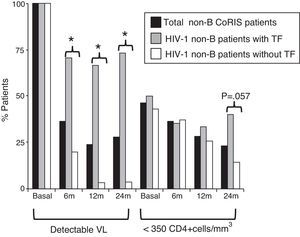
Differences in clinical-epidemiological features were assessed within the non-B CoRIS study group, according to the presence of TF. We observed that the 21 non-B CoRIS patients with TF showed significantly higher rates of infection by heterosexual contacts (86% vs. 61%) and had lower rates of syphilis diagnosis (0% vs. 22%) than those without TF (Table 2). Although with higher rates, there were not significant differences for tuberculosis and HCV. No associations were found for geographical origin, gender, age, educational level, clinical stage, HIV-1 infecting variant, VL or CD4 counts at recruitment time. As expected, VL values were significantly higher in those with TF after two years of first ART. However, we observed that 24 months after initiating ART, the rate of immunosuppressed patients (CD4+ <350 count) was higher among those with TF (40% vs. 14.3%, p=0.057) and close to significance (Fig. 2). Patients with and without TF have had the same mean time of follow-up (nearly 4 years) and number of visits (n=13), and was more frequent in those without TF (Table 3).
HIV-1 non-B-CoRIS patients with detectable VL and low CD4+, basal and months after first ART. *Significant difference, p<0.001; TF, treatment failure; VL, viral load; ART, antiretroviral therapy; detectable VL, >50 HIV-1-RNA copies/ml; low CD4+ levels, <350 CD4+cells/mm3. Only 55 of 82 non-B CoRIS patients (20 with TF and 35 without TF) had VL and CD4 data available at all time points.
Among the 57 non-B CoRIS subjects with available data regarding first ART, the mean time between enrollment and treatment initiation was 297 days (range 0–674 days). They had received up to 17 different ART. For the 21 patients with TF, only 20 had available data presenting 11 different first ART, the most frequently prescribed being emtricitabine (FTC)+tenofovir (TDF)+efavirenz (EFV), FTC+TDF+ lopinavir/ritonavir (LPV/r) and lamivudine (3TC)+zidovudine (AZT)+ saquinavir/ritonavir (SQV/r). When available, clinical reports showed that the main reason for the first change of ART among patients under TF was to present adherence failure or resistant viruses, followed by ART simplification and side effects or pregnancy. For the 34 of the 38 patients without TF and available first ART information, only 5 regimens were coincident with those with TF, FTC+TDF+EFV having been prescribed in 16 (47%) patients. Interestingly, considering ARV families involved in first ART, we found that patients with TF more frequently received first-line ART including protease inhibitors (PIs) than patients without TF (70% vs. 30%, p<0.05). TDR was present in 5 (9.4%) of the 53 non-B CoRIS naïve patients (only 1 in TF group) with available pol sequences at CoRIS enrollment (2 with K103N at RT, 2 with M46L and 1 with L90M, both at protease (PR).
DiscussionThe extensive variability of HIV-1 has a potential impact on epidemiology, diagnosis, therapy and prevention of infection.4 HIV-1 non-B variants, prevalent in the HIV/AIDS pandemic,1 are increasing in number and complexity in Western Europe,2 including in Spain,3,16 mainly due to emigration from countries endemic for these HIV-1 variants.17 However, non-B subtypes are not routinely well characterized and they are scarcely studied. The 82 non-B CoRIS cohort under study recruited from 2004 to 2008, represented the 12.8% of the complete CoRIS cohort at 2008. HIV-1 non-B-infected patients in Spain were more frequently women, immigrants (mainly from Sub-Saharan Africa), infected by the heterosexual route16,17 and by recombinant variants.3 The high percentage of women with non-B subtype found in our study supports previous reports showing that HIV-1 non-B-infected patients in Spain were more frequently women, immigrants and mainly from sub-Saharan Africa, where more than 58% of HIV-1 infected individuals are female and non-B variants are prevalent. It is in contrast to the HIV-infected native population and Latin Americans immigrants, who are more frequently men carrying subtype B.18,19
Here, we firstly observed that non-B CoRIS patients presented a high rate of TF, of losses to follow-up and more coinfections. We also found high level of basal CD4+ counts, whereas other studies have shown that the basal immune status was similar for different ethnic groups in CoRIS17 and in other European cohorts.20 These results could be explained as previous results supported a functional hierarchy of subtype B compared to other HIV-1 non-B subtypes for CD4 downregulation.21
The significantly high proportion of losses to follow-up described in non-B CoRIS group could be explained by the high proportion of immigrants with high CD4 levels, which have been previously associated to losses to clinical follow-up in the CoRIS cohort.11 Moreover, since many of non-B patients were foreigners, maybe they may have returned to their origin countries for care, explaining part of losses of follow-up. Nearly a third (32.9%) of non-B CoRIS HIV-1 patients were coinfected with other infectious agents, mainly tuberculosis (18%), HCV (16%) and syphilis (15.5%). Tuberculosis has been previously associated with low educational levels, African origin, heterosexual transmission and with an increased risk of AIDS,22 features found in the 8 HIV/tuberculosis coinfected subjects in the non-B CoRIS group. HIV/HCV coinfection, common among HIV-infected patients, increased the risk of death during our study period (2004–2008) in the whole CoRIS cohort.23 HIV/HCV coinfection prevalence was significantly lower in the non-B CoRIS group (16%) compared to that reported for the whole CoRIS cohort (37.3%)23 or for the EuroSIDA cohort (37%).24 Among HIV/HCV coinfected non-B CoRIS patients, only 3.4% were from Spain and 7% from other European countries. The HIV/syphilis coinfected non-B CoRIS patients (15.5%) were mainly men who have sex with men (MSM), Spaniards and Latin Americans, in agreement with previous studies.25 HIV/HBV coinfection was present in 2.9% of non-B CoRIS patients, which is lower than the 5% previously reported.26
The TDR rate, affecting first-line ART efficacy, has decreased in Spain in the last years,9,27 being higher in MSM and lower in injection drug users28 and is increasing among non-B-infected patients.8 Our study found 9.5% TDR prevalence for the non-B CoRIS study, similar to the 8.6% reported for the whole CoRIS cohort during 2007–2010 and lower than the 15.9% TDR rate for non-B variants in the cohort during the same period,16 where more pol sequences could be included for the analysis. In fact, resistance testing in naïve patients was not recommended in Spanish guidelines until early 2007.13 Although it has been reported that TDR rates could be higher in certain HIV-1 subtypes,6 similar TDR prevalence among B and non-B variants was found in other studies.29 However, considering ARV families, the found TDR prevalence in non-B CoRIS patients under study during 2004–2008 compared to that reported for the whole CoRIS cohort during 2007–201016 was similar for non-nucleoside reverse transcriptase inhibitors (NNRTIs) (3.8% vs. 3.9), lower for nucleoside reverse transcriptase inhibitors (NRTIs) (0% vs. 3.9%) and higher for PIs (5.7% vs. 2.3%). Thus, we observed that infection with non-B subtype variant reduced the risk of TDR to NRTIs and increased the risk of transmitted resistance to PIs, as in other studies.16 Interestingly, only one TF event could have been explained by TDR. Thus, despite TDR presence, the first ART in the remaining non-B CoRIS subjects carrying TDR was adequate.
The use of non-recommended regimens has been associated with a lack of virological response and higher mortality and has a greater impact for initial regimens containing NNRTIs.30 However, the TF rate found in the non-B CoRIS cohort was higher than the 13% described in the whole CoRIS cohort, where toxicity and not viral resistance was the main reason for an ART switch.31 Interestingly, our data reveals non-B CoRIS patients under TF presented a higher rate of heterosexual infections, HIV/tuberculosis and HIV/HCV coinfections and lower HIV/syphilis (absent) than those without TF experience. Furthermore, they more frequently received PIs based-first-ART, presenting lower CD4 counts after 24 months of first regimen. A recent study found that PIs (ATV/r) and NNRTIs (EFV) used as third drug had similar efficacy and safety in terms of virological response and CD4+ count increase in the CoRIS cohort.31 However, the higher TF rate observed in the non-B CoRIS cohort under PIs-based first ART in 65% of cases, can be due to the high rate of natural polymorphisms at PR codons related to drug resistance described in HIV-1 non-B variants,5,7 which can affect PIs susceptibility by restoring the replication capacity of resistance virus32 or by reducing the inhibitors binding affinity for the viral PR.
The main limitation of our study was the limited number of pol sequences (670) which could be recovered for HIV-1 variant characterization from the 3351 subjects recruited from 2004 to 2008 in the CoRIS cohort, since the Spanish guidelines only recommended resistance testing in naïve subjects as part of the clinical routine since 2007. Besides, when this study was performed, associated data were available at CoRIS cohort until October 2011. The second limitation was the lack of available information in the cohort's database for some studied variables, which could cause an underestimation of reported data. Furthermore, since treatment data were collected until 2011, the reported ART regimens could differ of actual regimens proposed in updated antiretroviral therapy guidelines. In conclusion, our results underline the particular epidemiologic and clinical profile of HIV-1 non-B-infected patients in Spain from 2004 to 2008. They were mostly immigrants, heterosexually infected, and mainly by recombinant viruses, with high prevalence of TF, losses to follow-up and the presence of coinfections, which may have important implications for the clinical management of HIV-1 non-B patients in Spain.
Funding sourcesThe RIS cohort (CoRIS) is funded by the Instituto de Salud Carlos III through the Red Temática de Investigación Cooperativa en Sida (ISCIII-RETIC RD06/006), by the RD12/0017/0018 project as part of the Plan Nacional R+D+ I and cofinanced by ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER).
This work was supported in part by grants from the Fondo de Investigaciones Sanitarias (FIS PI12/00240). E.T. is supported by the Centro de Investigación Biomédica en Red de Epidemiología y Salud Pública – CIBERESP.
Authors’ contributionsRR, JGG, FG, JLA, VA contribute to data collection. AH conceptualized and designed the study, reviewed and completed text, tables and figures. ET and TLLD performed the analyses and the final figures and tables. ET, GY and TLLD drafted the manuscript. All authors approved the final manuscript as submitted.
Conflict of interestThe authors confirm that this article content has no conflict of interest.
This study would not have been possible without the collaboration of all the patients, physicians, nurses, and data managers who have taken part in the project.
Steering Committee: Santiago Moreno, Julia del Amo, David Dalmau, Maria Luisa Navarro, Maria Isabel González, Vicente Soriano, Federico Garcia, Rafael Rubio, Jose Antonio Iribarren, Félix Gutiérrez, Francesc Vidal, Beatriz Hernández, Juan Berenguer.
Field work, data management and analysis: Paz Sobrino Vegas, Victoria Hernando Sebastián, Belén Alejos Ferreras, Débora Álvarez, Susana Monge, Inma Jarrín, Cristina González.
BioBank HIV: M Ángeles Muñoz-Fernández, Isabel García-Merino, Coral Gómez Rico, Jorge Gallego de la Fuente y Almudena García Torre.
Participating centres:
Hospital General Universitario de Alicante (Alicante): Joaquín Portilla, Esperanza Merino, Sergio Reus, Vicente Boix, Livia Giner, Carmen Gadea, Irene Portilla, Maria Pampliega, Marcos Díez, Juan Carlos Rodríguez, Jose Sánchez-Payá.
Hospital Universitario de Canarias (Santa Cruz de Tenerife): Juan Luis Gómez Sirvent, Patricia Rodríguez Fortúnez, María Remedios Alemán Valls, María del Mar Alonso Socas, María Inmaculada Hernández Hernández, Felicitas Díaz-Flores, Dácil García Rosado y Ricardo Pelazas González.
Hospital Carlos III (Madrid): Vicente Soriano, Pablo Labarga, Pablo Barreiro, Francisco Blanco, Luz Martín Carbonero, Eugenia Vispo, Carmen Solera, Carmen de Mendoza, Ana Treviño, Eva Poveda, Gustavo Manuzza, Lourdes Anta.
Hospital Doce de Octubre(Madrid): Rafael Rubio, Federico Pulido, Mariano Matarranz, Rafael Delgado, Asunción Hernando, Otilia Bisbal, Maria Lagarde, Guillermo Maestro, Rafael rubio-Martín.
Hospital Donostia(San Sebastián): José Antonio Iribarren, Julio Arrizabalaga, María José Aramburu, Xabier Camino, Francisco Rodríguez-Arrondo, Miguel Ángel von Wichmann, Lidia Pascual, Miguel Ángel Goenaga, Mª Jesús Bustinduy, Harkaitz Azkune Galparsoro, Maialen Ibarguren, Miriam Aguado.
Hospital General Universitario de Elche (Elche): Félix Gutiérrez, Mar Masiá, Sergio Padilla, Montserrat Ruiz, Andrés Navarro, Fernando Montolio, Catalina Robledano, Joan Gregori Colomé, Federico Carlos.
Hospital Gregorio Marañón (Madrid): Juan Berenguer, Juan Carlos López, Pilar Miralles, Isabel Gutiérrez, Margarita Ramírez, Belén Padilla, Paloma Gijón, Ana Carrero, Teresa Aldamiz-Echevarria, Francisco Tejerina, Fco José Parras, Pascual Balsalobre, Cristina Díez.
Hospital Universitario La Paz (Madrid): Juan González, Ignacio Bernardino de la Serna, José Ramón Arribas, María Luisa Montes, Jose Mª Peña, Blanca Arribas, Juan Miguel Castro, Francisco Javier Zamora, Ignacio Pérez, Miriam Estébanez, Silvia García-Bujalance, Natalia Stella,Jesús Mingorance.
Hospital de la Princesa (Madrid): Ignacio de los Santos, Jesús Sanz, Ana Salas, Cristina Sarriá, Ana Gómez.
Hospital San Pedro-CIBIR (Logroño): José Antonio Oteo, José Ramón Blanco, Valvanera Ibarra, Luis Metola, Mercedes Sanz, Laura Pérez-Martínez.
Hospital Universitario Mutua de Terrassa (Terrassa): David Dalmau, Angels Jaén, Mireia Cairó, Daniel Irigoyen, Laura Ibáñez, Queralt Jordano Montañez, Mariona Xercavins, Javier Martinez-Lacasa, Roser Font.
Complejo Hospitalario de Navarra (Pamplona): María Rivero, Itziar Casado, Jorge Díaz, Javier Uriz, Jesús Repáraz, Carmen Irigoyen, María Jesús Arraiza.
Hospital Parc Taulí (Sabadell): Ferrán Segura, María José Amengual, Gemma Navarro, Montserrat Sala, Manuel Cervantes, Valentín Pineda, Marta Navarro, Victor Segura.
Hospital Universitario Ramón y Cajal (Madrid): Santiago Moreno, José Luis Casado, Fernando Dronda, Ana Moreno, María Jesús Pérez Elías, Carolina Gutiérrez, Beatriz Hernández, María Pumares, Paloma Martí, Angel Lamas, Alberto de Diaz, Sergio Serrano, Lucas Donat.
Hospital San Cecilio (Granada): Federico García, José Hernández-Quero, Alejandro Peña, Leopoldo Muñoz, Jorge Parra, Vicente Guillot, Marta Alvarez, Natalia Chueca, David Vinuesa, Jose Angel Fernández.
Centro Sanitario Sandoval (Madrid): Jorge Del Romero, Carmen Rodríguez, Teresa Puerta, Juan Carlos Carrió, Paloma Raposo, Mar Vera, Juan Ballesteros.
Hospital Son Espases (Palma de Mallorca): Melchor Riera, Maria Peñaranda, Maria Leyes, Mª Angels Ribas, Antoni A Campins, Leire Gil, Francisco Fanjul, Carmen Vidal, Carmen Marinescu.
Hospital Virgen de la Victoria (Málaga): Jesús Santos, Manuel Márquez, Isabel Viciana Rosario Palacios.
Hospital Universitario Virgen del Rocío (Sevilla): Pompeyo Viciana, Manuel Leal, Luis Fernando López-Cortés, Mónica Trastoy, Pilar Pérez Romero.
Hospital Universitario La Fé (Valencia): Juan Cordoba, José Miguel Molina, Marta Montero, Marino Blanes, Miguel Salavert, José Lacruz.
Hospital Universitario de Santiago (Santiago de Compostela): Antonio Antela, Antonio Aguilera.
Hospital Universitari de Bellvitge (Hospitalet de Llobregat): Daniel Podzamczer, Elena Ferrerm Arkaitz Imaz, Evan Van Den Eyncle, Silvana Di Yacovo, Maria Sumoy.
Hospital de la Santa Creu i Sant Pau (Barcelona): Pere Domingo, Mª Antonia Sambeat, Karuna Lamarca, Gracia Mateo, Mar Gutiérrez, Irene Fernández.
Centro Colaborador: Centro Nacional de Microbiología (Majadahonda): Lucía Pérez Álvarez, Miguel Thomson Okatsu, Elena Delgado Blanco, Yolanda Vega Rocha.