Respiratory syncytial virus (RSV) is the main cause of severe bronchiolitis, especially in infants. The aim of this study is to assess whether codetection of RSV and other respiratory viruses could affect the severity of this infection comparing with unique RSV detection.
MethodsA prospective study from 2016 to 2019 including children under 2 years who were admitted in the Emergency Service of the Hospital Universitari Arnau de Vilanova de Lleida (Spain) was performed. Nasopharyngeal samples from all patients were sent to the laboratory for RSV real-time PCR detection (GeneXpert®). A multiplex PCR that detects other respiratory viruses was done in all RSV-positive samples. Patients’medical records were checked to collect clinical data (hospital length of stay, BROSJOD score, ICU admission, need for ventilatory support or transfer to a reference hospital). Patients were divided in two groups: infants with unique RSV detection and infants with viral codetection. Bivariant analyses were performed to analyze the data obtained.
ResultsDuring the period of study 437 RSV bronchiolitis were diagnosed. In 199 of them (177/437; 45,5%) another respiratory virus was detected concomitantly. Bivariant analyses do not show statistically significant differences between both groups.
ConclusionsViral codetection in infants with RSV bronchiolitis is frequent. However, it does not seems to affect the severity of this infection.
La bronquiolitis es una infección respiratoria grave en lactantes causada principalmente por el virus respiratorio sincitial (VRS). El objetivo de este estudio es evaluar si la codetección de VRS y otros virus respiratorios puede desencadenar un cuadro de mayor o menor gravedad que la presencia de VRS exclusivamente.
MétodosSe realizó un estudio prospectivo en menores de dos años que acudieron a Urgencias del Hospital Universitario Arnau de Vilanova de Lleida con diagnóstico de bronquiolitis aguda desde 2016 a 2019. A todos ellos se les realizó PCR de VRS y Gripe en muestra nasofaríngea (GeneXpert®) y a los pacientes con detección VRS se realizó PCR múltiple de virus respiratorios. Se recogieron datos clínicos de los pacientes (tiempo de hospitalización, índice de BROSJOD, ingreso en UCI, necesidad de soporte respiratorio, traslado a un centro de tercer nivel) y se realizaron análisis bivariantes para comparar el grupo de bronquiolitis con detección exclusiva de VRS con el de codetección con otros virus respiratorios.
ResultadosDurante el período de estudio se diagnosticaron 437 bronquiolitis por VRS. En 199 de ellas (199/437; 45,5%) se detectó concomitantemente otro virus respiratorio. El análisis bivariante de los datos no mostró diferencias estadísticamente significativas en ninguna de las variables estudiadas.
ConclusionesLa codetección viral en lactantes con bronquiolitis por VRS se da con elevada frecuencia, aunque no parece afectar a la gravedad del cuadro clínico.
Acute bronchiolitis is a viral infection typical of infants that affects the lower respiratory tract and causes obstruction and inflammation of the terminal bronchioles.1 Signs and symptoms usually start with rhinitis and cough, which can progress to tachypnoea, wheezing, rales, use of accessory muscles and/or nasal flaring.2,3 Most infants present mild disease, but hospital admission may be necessary in 2.5% of cases, mainly due to airway obstruction and respiratory distress,4 resulting in some 7000–14,000 hospital admissions per year in Spain.5 The main virus involved is the respiratory syncytial virus (RSV), although other respiratory viruses can also cause this condition.2,4,6
Viral co-detections in this type of infection are frequent, and their implication in the severity of the clinical picture is little studied, especially in RSV bronchiolitis. Several hypotheses have been proposed to explain the association between multiple respiratory viruses and the severity of acute respiratory infections, such as modified immune responses after initial infection, host susceptibility to multiple viral agents, and associations of specific respiratory viruses with bacteria colonising the respiratory tract.7 In addition, it should be noted that detecting a viral agent does not necessarily imply its participation in the current infectious process.8
The aim of this study was to assess whether patients with viral co-detection in RSV bronchiolitis presenting at the emergency department have a better or worse clinical prognosis than those with exclusive RSV detection.
MethodsPatients included in the studyThis was a prospective study that included all infants under two years of age who attended the emergency department of the Arnau de Vilanova de Lleida University Hospital with a diagnosis of bronchiolitis from 1 November 2016 to 30 April 2019, in whom RSV was detected in a nasopharyngeal sample.
Acute bronchiolitis was considered the first episode of respiratory distress in a child under 24 months of age with wheezing and/or crackles associated with upper respiratory tract symptoms during the epidemic period.9
Detection of respiratory virusesA nasopharyngeal sample was taken from each patient with a swab in a standard virus transport medium (Universal Virus Transport®, Becton Dickinson, Franklin Lakes, USA) of 1 or 3ml. The samples were sent to the Microbiology Laboratory to carry out molecular analysis. Detection of influenza A (Flu A), influenza B (Flu B) and RSV viruses was performed urgently with real-time multiplex PCR (GeneXpert®, Xpert®Flu/RSV XC, Cepheid Inc., Sunnyvale, USA).
In all the patients in whom RSV was detected, the molecular study of respiratory viruses was extended. For this, 60 μl were extracted by elution from 200 μl of sample (Virus Mini Kit v2.0, EZ1® kit [Qiagen GmbH, Germany] or DSP Virus/Pathogen Minikit, QIAsymphony® system [Qiagen GmbH, Germany], according to the manufacturer's instructions.
From 2016 to the first half of 2018, the detection of the rest of the respiratory viruses was performed with the Allplex® Respiratory Panel (Seegene, Seoul, South Korea) in the CFX96® thermal cycler (Bio-Rad Laboratories, Hercules, USA). This technique detects adenovirus (AdV), enterovirus (EV), parainfluenza virus (PiV) 1, 2, 3 and 4, metapneumovirus (MPV), bocavirus (BoV), rhinovirus (HRV) and coronavirus (CoV) NL63, 229E and OC43. As of the second half of 2018 and during 2019, it was performed with the Light Mix® kit (TIB Molbiol, Berlin, Germany) in the LightCycler® 480 II thermocycler (Roche Diagnostics, Penzberg, Germany), which includes, in addition to those previously mentioned, parechovirus (PeV) and CoV in a generic way, differentiating the HKU1 and MERS subtypes.
Data collectionThe clinical histories of patients diagnosed with acute RSV bronchiolitis were reviewed and the following clinical and microbiological data were collected: results of the detection of the rest of the respiratory viruses, age, bronchiolitis score of Sant Joan de Déu (BROSJOD),10 duration of hospitalisation (time spent by the patient in the hospital, including the emergency department, observation and ward), need for oxygen therapy, days of oxygen treatment, maximum concentration of fraction of inspired oxygen (FiO2) required, need for respiratory support or mechanical ventilation, admission to a paediatric intensive care unit (PICU), need for nasogastric tube (NGT) feeding, need for transfer to a tertiary care centre, and administration of palivizumab prior to admission. This study was approved by the ethics committee of the Hospital Universitari Arnau de Vilanova.
Statistical analysisThe descriptive analysis included the median and interquartile interval for the quantitative variables, and the absolute and relative frequencies for the qualitative ones.
Bivariate analyses were performed to compare the two groups of patients based on viral detection in nasopharyngeal swabs (RSV only or viral co-detection) and two age groups of infants (younger than and older than 3 months). For this, the chi-square test was used for categorical variables and the Mann-Whitney U test for quantitative variables. A logistic regression model was created to estimate the association of the presence of mixed infections in relation to age, allowing for non-linear relationships. For the statistical analysis, the R214 software was used, applying a significance level of 0.05.
ResultsDetection of respiratory viruses in infants with RSV bronchiolitisDuring the study period, 437 episodes of RSV bronchiolitis were diagnosed in 435 infants. In 199 of the 437 samples (45.5%) another viral agent was detected in addition to RSV.
The main viruses co-detected were: HRV (83/199; 19%), BoV (75/199; 17.2%), AdV (41/199; 38%), CoV (23/199; 5.26%), PiV (10/199; 2.29%), EV (6/199; 1.37%), Flu A (5/199; 1.14%) (4 H3 subtype and 1 H1 subtype), Flu B (3/199; 0.69%) and MPV (4/199; 0.92%). PeV detection was performed only in the last year and was detected in a single episode (1/199; 0.23%). Two viruses other than RSV were detected in 47 episodes (10.75%) and three viruses in five episodes (1.14%). The virus most frequently detected in these multiple detections was AdV (20/41), followed by CoV (11/23) and BoV (35/75).
Clinical and epidemiological dataOverall, 52.9% of RSV bronchiolitis (231/437) occurred in patients under three months of age. The mean length of hospital stay (both in the emergency room and observation room or ward) was five days, and 62.9% (275/437) of the patients required oxygen supplementation, with a mean duration of one day. The maximum FiO2 required was 27% on average.
In all, 20.9% of the episodes (91/437) required NGT feeding, 21.6% (94/437) respiratory support, 29.5% (129/437) admission to the neonatal ICU, and 5% (22/437) transfer to a tertiary care centre
Regarding the severity of the bronchiolitis, 72 episodes (16.5%) presented a mild BROSJOD, 320 (73.2%) moderate, and 45 (10.3%) severe. There were no cases of death or history of receiving palivizumab prophylaxis.
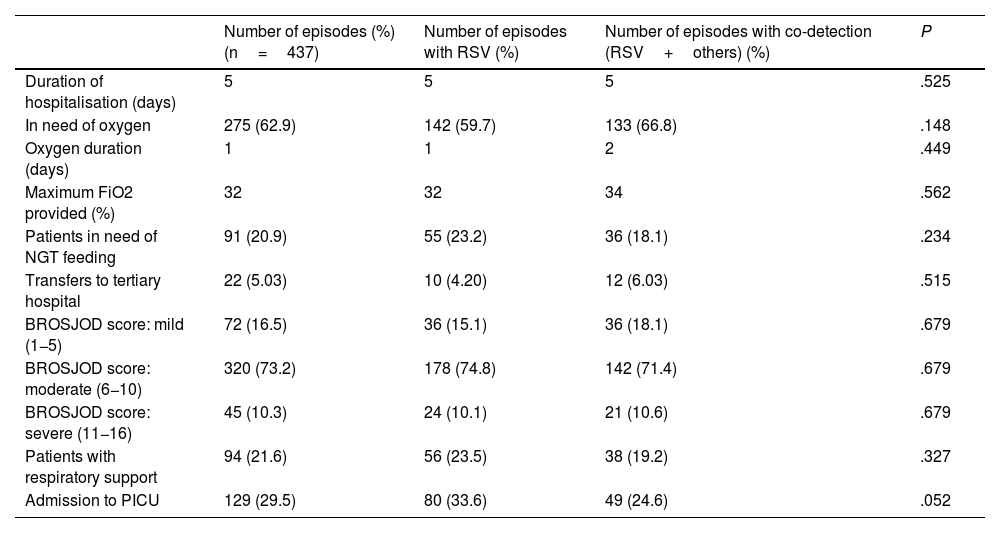
Table 1 shows the characteristics of episodes of bronchiolitis with single detection of RSV and those with co-detection with other respiratory viruses. No statistically significant differences between these two groups were found in any of the variables studied (P>.05).
Characteristics of RSV bronchiolitis episodes.
| Number of episodes (%) (n=437) | Number of episodes with RSV (%) | Number of episodes with co-detection (RSV+others) (%) | P | |
|---|---|---|---|---|
| Duration of hospitalisation (days) | 5 | 5 | 5 | .525 |
| In need of oxygen | 275 (62.9) | 142 (59.7) | 133 (66.8) | .148 |
| Oxygen duration (days) | 1 | 1 | 2 | .449 |
| Maximum FiO2 provided (%) | 32 | 32 | 34 | .562 |
| Patients in need of NGT feeding | 91 (20.9) | 55 (23.2) | 36 (18.1) | .234 |
| Transfers to tertiary hospital | 22 (5.03) | 10 (4.20) | 12 (6.03) | .515 |
| BROSJOD score: mild (1−5) | 72 (16.5) | 36 (15.1) | 36 (18.1) | .679 |
| BROSJOD score: moderate (6−10) | 320 (73.2) | 178 (74.8) | 142 (71.4) | .679 |
| BROSJOD score: severe (11−16) | 45 (10.3) | 24 (10.1) | 21 (10.6) | .679 |
| Patients with respiratory support | 94 (21.6) | 56 (23.5) | 38 (19.2) | .327 |
| Admission to PICU | 129 (29.5) | 80 (33.6) | 49 (24.6) | .052 |
FiO2: fraction of inspired oxygen; NGT: nasogastric tube; PICU: paediatric intensive care unit; RSV: respiratory syncytial virus.
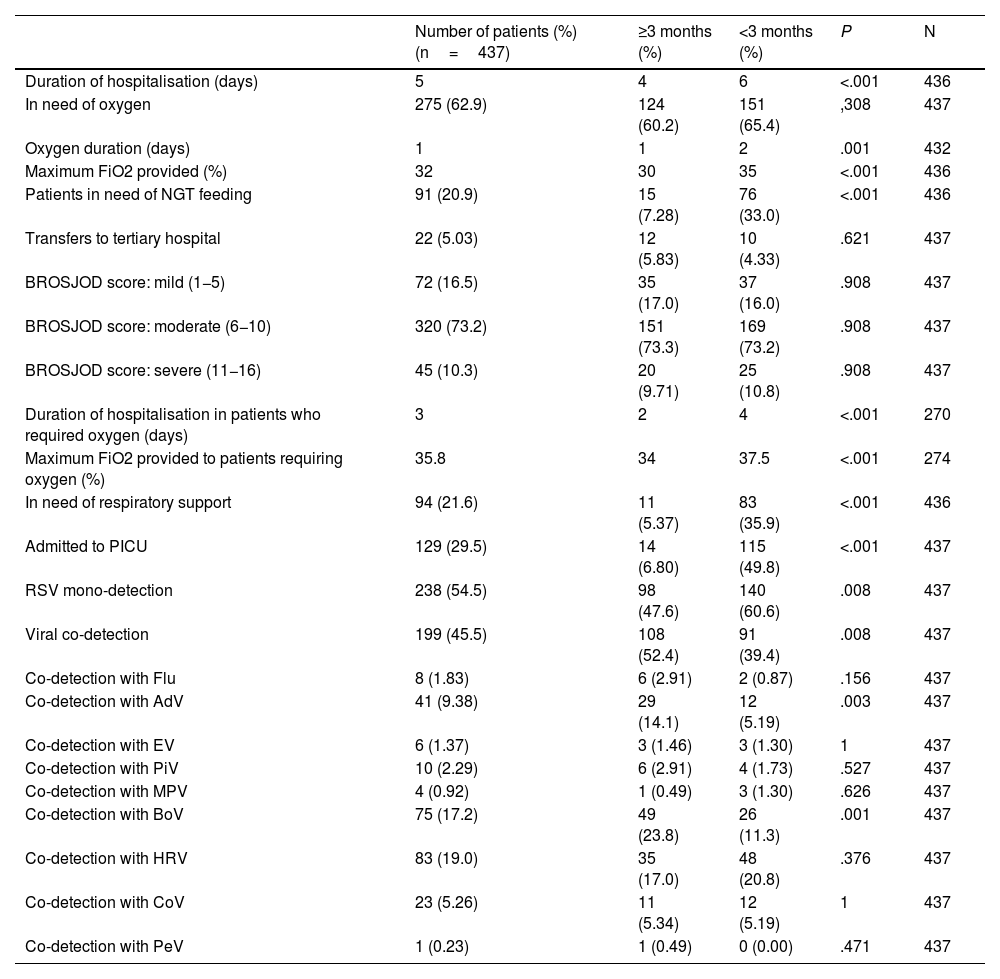
When comparing the characteristics of infants younger than and older than three months, statistically significant differences were found (Table 2). Infants under three months old had a longer hospital stay (six days vs. four days, P<.001), longer duration of oxygen administration (two days vs. one day, P=.001), a higher requirement of maximum FiO2 (35% vs. 30%, P<.001), greater need for NGT (33.0% vs. 7.3%, P≤.001), greater need for respiratory support (35.9% vs. 5.4%, P<.001) and greater admission to the PICU (49.8% vs. 6.8%, P<.001).
Characteristics of patients with RSV bronchiolitis younger than 3 months compared with patients 3 months or older.
| Number of patients (%) (n=437) | ≥3 months (%) | <3 months (%) | P | N | |
|---|---|---|---|---|---|
| Duration of hospitalisation (days) | 5 | 4 | 6 | <.001 | 436 |
| In need of oxygen | 275 (62.9) | 124 (60.2) | 151 (65.4) | ,308 | 437 |
| Oxygen duration (days) | 1 | 1 | 2 | .001 | 432 |
| Maximum FiO2 provided (%) | 32 | 30 | 35 | <.001 | 436 |
| Patients in need of NGT feeding | 91 (20.9) | 15 (7.28) | 76 (33.0) | <.001 | 436 |
| Transfers to tertiary hospital | 22 (5.03) | 12 (5.83) | 10 (4.33) | .621 | 437 |
| BROSJOD score: mild (1−5) | 72 (16.5) | 35 (17.0) | 37 (16.0) | .908 | 437 |
| BROSJOD score: moderate (6−10) | 320 (73.2) | 151 (73.3) | 169 (73.2) | .908 | 437 |
| BROSJOD score: severe (11−16) | 45 (10.3) | 20 (9.71) | 25 (10.8) | .908 | 437 |
| Duration of hospitalisation in patients who required oxygen (days) | 3 | 2 | 4 | <.001 | 270 |
| Maximum FiO2 provided to patients requiring oxygen (%) | 35.8 | 34 | 37.5 | <.001 | 274 |
| In need of respiratory support | 94 (21.6) | 11 (5.37) | 83 (35.9) | <.001 | 436 |
| Admitted to PICU | 129 (29.5) | 14 (6.80) | 115 (49.8) | <.001 | 437 |
| RSV mono-detection | 238 (54.5) | 98 (47.6) | 140 (60.6) | .008 | 437 |
| Viral co-detection | 199 (45.5) | 108 (52.4) | 91 (39.4) | .008 | 437 |
| Co-detection with Flu | 8 (1.83) | 6 (2.91) | 2 (0.87) | .156 | 437 |
| Co-detection with AdV | 41 (9.38) | 29 (14.1) | 12 (5.19) | .003 | 437 |
| Co-detection with EV | 6 (1.37) | 3 (1.46) | 3 (1.30) | 1 | 437 |
| Co-detection with PiV | 10 (2.29) | 6 (2.91) | 4 (1.73) | .527 | 437 |
| Co-detection with MPV | 4 (0.92) | 1 (0.49) | 3 (1.30) | .626 | 437 |
| Co-detection with BoV | 75 (17.2) | 49 (23.8) | 26 (11.3) | .001 | 437 |
| Co-detection with HRV | 83 (19.0) | 35 (17.0) | 48 (20.8) | .376 | 437 |
| Co-detection with CoV | 23 (5.26) | 11 (5.34) | 12 (5.19) | 1 | 437 |
| Co-detection with PeV | 1 (0.23) | 1 (0.49) | 0 (0.00) | .471 | 437 |
AdV: adenovirus; BoV: bocavirus; CoV: coronavirus; EV: enterovirus; FiO2: fraction of inspired oxygen; Flu: influenza virus; HRV: human rhinovirus; MPV: metapneumovirus; PeV: parechovirus; PiV: parainfluenza virus; NGT: nasogastric tube; PICU: paediatric intensive care unit; RSV: respiratory syncytial virus.
Regarding the detection of other viruses, infants under three months of age had fewer co-detections overall (39.4% vs. 52.4%, P=.008). Depending on the viral agent, they presented fewer AdV (5.19% vs. 14.1%, P=.003) and BoV (11.3% vs. 23.8%, P=.001) co-detections than the group of older infants. In this group, the presence of more than two viruses was detected in 18.68% of the episodes (17/91) compared to the group older than three months, where it was 27.78% (30/108).
DiscussionThe prevalence of viral co-detection in infants with RSV bronchiolitis in this study was close to 50%. This figure is much higher than that described in other similar studies, which report values between 11.33% and 24.4%,11–14 reaching up to 29.8% in severe bronchiolitis due to RSV.15 A study carried out in Spain detected a 35.8% viral co-detection in infants under 18 months of age with RSV bronchiolitis,16 a value closer, although lower, to that detected in our study. The large differences between the results of these studies could be explained by the use of different PCR techniques, different populations studied and different geographical areas.
The results obtained in our study do not show significant differences between the group of patients with bronchiolitis with mono-detection of RSV and the patients with co-detection of RSV and another respiratory virus in terms of the severity factors evaluated, in agreement with those obtained in similar studies.11,17–19 A study carried out by Nascimiento et al. in infants under two years of age who presented to the emergency department with bronchiolitis concluded that the sole presence of RSV was associated with a greater risk of hospital admission, but co-detections were not associated with greater clinical severity or ICU admission.20
Likewise, a systematic review and meta-analysis carried out in 2016 on viral co-detections in children under five years of age hospitalised with respiratory viral infection21 showed no differences in ICU admission, oxygen use, mechanical ventilation or radiological abnormalities between the group of children with mono-detection and the one with co-detection. In that same year, another meta-analysis concluded that viral coinfection did not increase the risk of any of the factors evaluated in patients under 18 years of age with acute respiratory infection.22 A systematic review published in 2013 also did not associate an increase in the severity of cases of viral codetection in children under six years of age.23
However, Midulla et al. reported that infants with RSV and BoV co-detection had greater severity and more days of hospital stay than those with RSV, HRV or BoV mono-detection.24 Resch et al. analysed 745 serious RSV infections (70% bronchiolitis) in a mainly infant population, concluding that patients with viral co-detection had required higher rates of supplemental oxygen and respiratory support.25 Other authors analyse bronchiolitis due to any viral agent, concluding that patients with viral co-detection had a 2.7 times greater risk of admission to the ICU than those with exclusive detection of a single viral agent.13
On the other hand, there are studies that relate greater severity in RSV bronchiolitis exclusively compared to bronchiolitis in which other respiratory viruses are also detected. Marguet et al. observed a longer length of hospital stay in those with RSV mono-detection compared to those with RSV/HRV co-detection.12 Another study, carried out in Italy, concluded that RSV mono-detection led to more hospitalisation time and more hypoxia than MPV/RSV co-detection in infants hospitalised for acute respiratory disease.26
Regarding the mean hospitalisation time, no statistically significant differences were found in our study between infants in whom only RSV was detected and those who presented viral co-detection. However, a shorter duration of hospital stay was observed for infants admitted for RSV bronchiolitis than in other studies published in Spain (5 days vs. 5.9 days),27 which was well below that of another study conducted in Austria on severe RSV infections (6.7 days on average).25 However, other studies reflect much shorter mean hospitalisation times (2–3 days in a multicentre study conducted in the USA15). The mean hospitalisation time is a subjective parameter that depends on the physician and the department in which the patient is admitted, as well as the hospital and the criteria for considering a patient as hospitalised. In our study, the entire time that the patient remained in the hospital was considered as the hospital stay, regardless of whether they remained in the emergency department, observation or ward. Therefore, it is not a consistent parameter to compare our data with other hospitals or studies carried out.
Among the strengths of our study, we point out its prospective design, its duration and the considerable number of patients included, all of them infants with RSV bronchiolitis.
The main limitations derive from the respiratory virus detection techniques themselves (real-time PCR). The fact of detecting more than one viral agent does not necessarily imply the participation of the pathogens in the infectious process, since the presence of genetic material can be detected up to five or six weeks later in some cases.8 On the other hand, there may be a low recovery of viral agents and underestimation of positive samples due to factors such as delayed sampling, since the viral load decreases over time.28,29 It should be noted that there is great methodological variability (different age groups, different geographical locations, different PCR techniques used, etc.) in published studies on the clinical relevance of viral co-detection in patients with respiratory infection, which makes it difficult to compare the results obtained.
In conclusion, there does not appear to be greater severity among infants with RSV bronchiolitis and those with RSV plus another viral agent. Nevertheless, we consider it necessary to carry out more prospective multicentre studies with a common methodology in order to better define the clinical relevance of viral co-detection in this type of patient.
Conflicts of interestThe authors declare that they have no conflicts of interest.