Although obligate anaerobes are seldom isolated from patients with bacteraemia, the genus Clostridium is in second place behind the genus Bacteroides and represents approximately 1% of all positive blood cultures, with Clostridium perfringens being the most commonly isolated species. The risk factors associated with its isolation in blood are haemolysis, malignant intestinal neoplasia, inflammatory bowel disease and immunosuppression.1 In most cases, its clinical meaning is unclear, representing contamination or transient bacteraemia, and its pathogenicity and virulence continue to be a subject of debate. Clostridium colicanis is a Clostridium species that has been rarely isolated in the blood, the first time in 2008 by Simmon et al., although it was not documented the episode.2 Thus, we report the first documented case of bacteraemia by this microorganism in an immunocompetent patient diagnosed as acute respiratory infection.
We present the case of a 77-year-old woman with asthma and anticoagulation who was admitted to the Emergency Department due to symptoms of fever of up to 39°C, chills, malaise, asthenia, dyspnoea, cough and decreased level of consciousness. The abdominal anamnesis was anodyne, and the patient presented no urinary symptoms, heart failure or oedema. The physical examination revealed a blood pressure of 120/63mm Hg, a temperature of 37.7°C and an oxygen saturation of 95%. The most noteworthy laboratory data were as follows: leukocytes count of 23.7×109/L [4–11] with 89.4% [40–80] granulocytes and 4.6% [20–50] lymphocytes, prothrombin activity of 42% [70–120], international normalised ratio of 1.85 [0.8–1.85], total bilirubin of 1.7mg/dL [0.2–1.2] and C-reactive protein of 41.9mg/L [0–5]. Upon her arrival, the patient underwent blood cultures, influenza A/B virus detection using polymerase chain reaction in a nasopharyngeal exudate (negative) and urine culture (negative). Treatment was started with intravenous cefotaxime (1g/8h for 10 days) and oral levofloxacin (500mg/day for 7 days), which resulted in the disappearance of the fever.
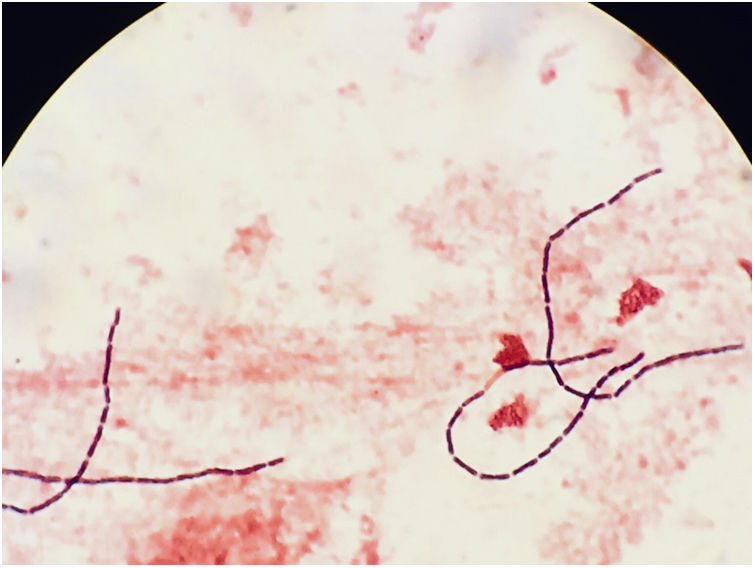
The blood cultures were processed in the BD BACTEC™ 9240 system (Becton-Dickinson and Company, NJ, USA). The two anaerobic bottles were positive after 26h of incubation. Gram staining revealed the presence of long Gram-positive bacilli with straight ends (Fig. 1), which were isolated under anaerobic conditions (Oxoid™ AnaeroGen™ 2.5-L sachet, ThermoFisher Scientific) in Schaedler agar at 48h. The colonies were round, somewhat irregular, white-grey, catalase-negative measuring approximately 3mm in diameter. The strain was identified as C. colicanis (log score: 2.122) using matrix-assisted laser desorption ionisation time of flight mass spectrometry (MALDI Biotyper® Microflex LT, Bruker Daltonik GmbH), and 16S rRNA sequencing (99%, GenBank accession number FJ957867.1). Antimicrobial susceptibility testing was carried out by the Etest gradient diffusion method (bioMérieux, Marcy ĺetoile, France) using a 0.5 McFarland bacterial suspension and Brucella blood agar with hemin and vitamin K1. The plates were incubated anaerobically for 48h at 35–37°C. The minimum inhibitory concentration (μg/mL) was interpreted as susceptible according to the recommendations for anaerobic bacteria (EUCAST and CLSI criteria)3,4: penicillin (0.016), amoxicillin-clavulanic acid (0.094), cefotaxime (0.015), piperacillin-tazobactam (0.016), clindamycin (2), metronidazole (1), meropenem (0.002) and tetracycline (1.5). After isolating C. colicanis, an abdominal ultrasound was requested, which showed no significant abnormalities. The patient progressed favourably and was discharged from the hospital.
C. colicanis is a bacillus measuring approximately 0.9–1.0×3–10μm and is Gram-positive, obligate anaerobic, sporulating, nonmotile, and catalase-negative. It can use a considerable number of substrates, producing various acids from glucose, lactose, maltose, mannose, ribose, cellobiose and galactose and can reduce nitrates to nitrites.5 Its genome consists of a single chromosome (2.6 Mpb) and contains approximately 2160 protein-encoding genes.6 The colonies measure 3–5μm in diameter and are round with rippled edges, slightly convex, opaque and white-grey. Its optimal growth temperature is 37–40°C.
This microorganism was first described after its isolation in the faeces of a male Labrador dog in 2003.5 The microorganism is closely related phylogenetically with C. absonum, C. baratii and Eubacterium multiforme. C. colicanis bacteraemia was first reported in humans in 2008 in a scientific article that studied the genotypic diversity of anaerobic isolates from bloodstream infections.2 This bacillus was subsequently encountered in 2014, in a study that compared the faecal microbiota of 13 Thai vegetarians and nonvegetarians and was found in a 61-year-old vegetarian who did not eat either yoghourt or eggs but did drink milk.7 A recent study reported that, in more than half of patients with gastric cancer, the most prevalent microorganisms in the gastric epithelium were bacteria of the species Fusobacterium nucleatum (whose pathogenic role in colorectal cancer is well-known) and C. colicanis, suggesting a possible contribution of these bacteria in the development or progression of stomach cancer.8 Our case corresponded to transient bacteraemia in a patient with laboratory data suggesting infection, and to date no signs of gastric or colon neoplasia have been found.
In conclusion, we reported the first documented case of C. colicanis bacteraemia in an immunocompetent patient, highlighting the importance of C. colicanis as a human pathogen. Further studies are needed to elucidate the pathogenesis and risk factors of C. colicanis-related invasive infections such as bacteraemia.
FundingNone.
Conflicts of interestThe authors declare no conflicts of interest.
Thanks to Carmen Martín Salas of the Servicio de Microbiología Clínica, Complejo Hospitalario de Navarra for her help in drafting the manuscript.