To assess the correlation of procalcitonin (PCT), C-reactive protein (CRP), neopterin, mid-regional pro-atrial natriuretic peptide (MR-proANP), and mid-regional pro-adrenomedullin (MR-proADM) with severity risk scores: severe CAP (SCAP) and SMART-COP in patients with community-acquired pneumonia (CAP), as well as short term prognosis and to determine the correlation with mortality risk scores.
MethodsEighty-five patients with a final diagnosis of pneumonia were consecutively included during a two month period. Epidemiological, clinical, microbiological, and radiological data were recorded. Patients were stratified according to the PSI, CURB-65, SCAP and SMART-COP. Complications were defined as respiratory failure/shock, need of ICU, and death. Plasma samples were collected at admission.
ResultsMR-proANP and MR-proADM showed significantly higher levels in high risk SCAP group in comparison to low risk. When considering SMART-COP none of the biomarkers showed statistical differences. MR-proADM levels were high in patients with high risk of needing intensive respiratory or vasopressor support according to SMRT-CO. Neopterin and MR-proADM were significantly higher in patients that developed complications. PCT and MR-proADM showed significantly higher levels in cases of a definite bacterial diagnosis in comparison to probable bacterial, and unknown origin. MR-proANP and MR-proADM levels increased statistically according to PSI and CURB-65.
ConclusionsBiomarker levels are higher in pneumonia patients with a poorer prognosis according to SCAP and SMART-COP indexes, and to the development of complications.
Establecer la correlación entre los niveles de procalcitonina (PCT), proteína C reactiva, neopterina, pro-péptido natriurético auricular (MR-proANP) y pro-adrenomedulina (MR-proADM) y los índices de severidad: severe CAP (SCAP) y SMART-COP en pacientes con neumonía adquirida en la comunidad (NAC), así como el pronóstico a corto plazo, y confirmar su correlación con los índices de severidad PSI y CURB-65.
MétodosOchenta y cinco pacientes con diagnóstico final de NAC fueron incluidos de forma consecutiva durante 2 meses. Se recogieron los datos epidemiológicos, clínicos, microbiológicos y radiológicos. Los pacientes se clasificaron en función del PSI, CURB-65, SCAP y SMART-COP. Las complicaciones se definieron como insuficiencia respiratoria/shock, ingreso en la UCI o muerte. Las muestras de plasma se recogieron en el momento del ingreso hospitalario.
ResultadosLos niveles de MR-proANP y MR-proADM fueron significativamente superiores en aquellos pacientes clasificados como alto riesgo según SCAP en comparación con los de bajo riesgo. Al considerar SMART-COP ninguno de los biomarcadores mostró significación estadística. Los niveles de MR-proADM fueron superiores en los pacientes con alto riesgo de necesitar soporte intensivo/vasopresor según SMRT-CO. Los valores de neopterina y MR-proADM fueron significativamente superiores en pacientes que desarrollaron alguna complicación. En los casos con diagnóstico bacteriano de seguridad, se observaron niveles significativamente más elevados de PCT y MR-proADM, respecto de los casos de probable origen bacteriano o origen desconocido. Los niveles de MR-proANP y MR-proADM se incrementaron en función del PSI y de CURB-65.
ConclusionesLos niveles de biomarcadores son superiores en pacientes con peor pronóstico, según los índices de severidad evaluados, así como con el desarrollo de complicaciones.
The optimal management of community-acquired pneumonia (CAP) requires clinical decisions regarding the initial site of care and therapy. Appropriate decisions in this setting are important for an adequate patient care and correct allocation of resources.
Regarding severity assessment, several prognostic scores have been developed in order to assess the risk of death, such as the Pneumonia Severity Index (PSI)1 and CURB-65 (confusion, urea, respiratory rate, blood pressure and age).2 In general, severity rules consider several clinical, analytical and radiological findings that jointly reflect patient's general condition. Although these rules can be useful for the management of patients with pneumonia, they also present some disadvantages such as age overemphasis and complexity for its calculation. In the last years, two other severity scores have been defined: severe CAP (SCAP) that was developed for identifying patients who are at risk for an adverse outcome and might need ICU admission, being as accurate as current scoring systems3–5 and SMART-COP, mainly designed for the prediction of patients that are likely to require intensive respiratory or vasopressor support (IRVS).6 Main drawbacks for these last scores are the lack of consideration for the presence of comorbidities and the need of more testing and validation, although results from a recent meta-analysis indicate their usefulness for the prediction of ICU admission or intensive treatment in patients with CAP.7 In a study with patients aged <50, SMART-COP was superior to PSI and CURB-65 for the prediction of IRVS, but incorrectly stratified 15% of patients.8
In the last years, it has also become more evident that it is also important to consider host inflammatory and cardiovascular response to an infection.9 Procalcitonin (PCT), C-reactive protein (CRP) and neopterin are examples of biomarkers that can be useful for the management of patients with pneumonia, as a correlation with the etiological origin and the severity has been demonstrated.10,11 Biomarkers reflecting cardiovascular impairment (including endothelial dysfunction and volume homeostasis) have also emerged as useful tools for pneumonia management.12,13 Adrenomedullin (ADM) is a member of the CALC-gene family and has potent vasodilating, immune modulating and metabolic properties.14 Atrial natriuretic peptide (ANP) is synthesized by cardiac atrial myocytes in response to proinflammatory factors, hypoxia and conditions of increased cardiac pressure and volume overload.15 Biochemical assays aim specifically at the mid-region of the ADM and ANP precursors (MR-proADM and MR-proANP).16,17 Levels of both biomarkers have been evaluated as severity and prognostic markers in CAP and chronic obstructive pulmonary disease (COPD) exacerbations correlating with PSI, CURB-65, the simpler CRB-65 and prognosis.18–23 Indeed these biomarkers have shown to improve usefulness of validated scores.24,25 However, little is known about how these biomarkers correlate with the severity indexes: SCAP and SMART-COP.
Inflammatory and cardiovascular biomarkers have shown to correlate to some extent with etiology, severity of CAP and to mortality risk scores.13,26 Therefore, we hypothesized that biomarkers should also correlate to severity scores primary aimed to identify patients needing intensive care and even improve its usefulness. Therefore, the main objective of this study was to assess the correlation of PCT, CRP, neopterin, MR-proANP and MR-proADM levels with mortality risk scores, focusing on SCAP and SMART-COP. The secondary objectives were to confirm the correlation of biomarkers with short term mortality and to evaluate its usefulness for identifying bacterial etiology.
Patients and methodsStudy design and settingThe study is observational, descriptive and analytical and was approved by the ethical committee of the institution. Population consists of patients attending a tertiary public university hospital with fever and symptoms of lower respiratory tract infection (LRTI) that consulted the medical area of the emergency department (ED) (excluding surgical, gynecological and pediatric areas) and from whom blood cultures were obtained. Patients were consecutively included during two months period. Patients were followed up for 30 days after admission. Pneumonia was defined by clinical (presence of fever, cough and dyspnea) and radiographic signs (pneumonic infiltrate in the chest radiograph), as well as clinical evolution, assessed by expert clinicians and radiologists.27 Final diagnosis was set according to the clinical judgment mentioned in the emergency and hospital medical files, or in the records of outpatient care. For doubtful cases, a consensus was achieved by three expert clinicians. People conducting the chart abstraction, and reviewing chest X-rays were blinded to the study hypothesis and blinded to biomarkers values.
Data collection and sample processingEpidemiological, clinical, microbiological, analytical and radiological data were recorded from all cases. Charlson index was also calculated for each patient.28 Patients were stratified according to the PSI, CURB-65, SCAP and SMART-COP.1,2,5,6 SMART-COP was calculated if all variables were available. SMRT-CO was applied in cases when either one of the following variables was not recorded: albumin, arterial pH, or Pa O2. Complications considered were: respiratory failure (Pa O2<60mmHg), shock (hypotension persisting despite fluid resuscitation and requiring vasopressor therapy),29 need of ICU admission, and death.
At the time of arrival to the ED, samples were collected for microbiological diagnosis: blood cultures, respiratory specimens for culture and urine for antigen detection. Pneumococcal pneumonia was diagnosed by isolation of Streptococcus pneumoniae from blood and/or pleural effusion culture and/or detection of C-polysaccharide antigen by inmunochromatography (ICT) (Binax Now S. pneumoniae urinary antigen test, Binax. Maine, USA).30 In COPD patients, because of the lack of specificity of ICT,31 detection of polysaccharide capsular antigen was performed by counterimmunoelectrophoresis.32Legionella pneumonia was diagnosed by urinary antigen detection of Legionella pneumophila serogroup 1 by enzyme immunoassay (Bartels EIA Legionella urinary antigen, Trinity Biotech Company, Ireland) or microorganism isolation in a respiratory sample. Definite bacterial origin also included cases with isolation of a microorganism different from S. pneumoniae from blood culture and/or pleural effusion culture. The isolation of a predominant microorganism in the sputum samples was considered as probable etiology and not as a definite diagnosis.
Plasma samples were collected at ED admission, together with first routine hemogram. All samples were stored at −20°C until biomarkers measurements. CRP was measured by turbidimetric assay (RCRP, Siemens Dimension Rxl Max, Siemens, Germany). PCT, MR-proANP and MR-proADM were measured with an immunofluorescent assay (KRYPTOR BRAHMS AG, Germany) and neopterin levels with a competitive immunoassay following the manufacturer's instructions (Neopterin ELISA, IBL, Germany).
Statistical analysisCategorical variables are expressed as counts (percentages) and continuous variables as mean and standard deviation (SD) or as median and interquartile range (IQR), as appropriate. Biomarkers levels are expressed as median and IQR. Correlation analysis was performed using Spearman's correlation. The χ2-test was used to compare categorical variables. In case of quantitative variables, Mann–Whitney U-test and Kruskal–Wallis were used as appropriate. To assess the accuracy of biomarkers for predicting the development of complications, we performed receiver operating characteristic curves and determined the area under the curve (AUC), standard error (SE) and 95% confidence interval (95% CI). Associations were considered statistically significant if p value<0.05. The commercial statistical software package used was SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
ResultsSamples from 162 patients were collected and after retrospective analysis, 85 patients were considered to have presented with pneumonia. Alternative diagnoses were COPD exacerbation (25 patients) and other bronchial infections without pneumonic infiltrate in chest X-ray (52 patients). In Table 1 biomarker levels in the three study groups are shown. PCT (p=0.001), CRP (p=0.008) and MR-proADM (p=0.006) showed significant differences when comparing levels in the three groups while no differences were found for MR-proANP and neopterin. Specifically, PCT showed significantly higher levels in pneumonia when comparing with COPD exacerbation (p=0.003) and bronchial infection (p=0.002). Levels of PCT were similar in COPD exacerbation and bronchial infection groups (p=0.446). CRP only showed significantly higher levels when comparing pneumonia and bronchial infection groups (p=0.002). Finally, MR-proADM showed statistical higher levels when comparing pneumonia group with COPD exacerbation (p=0.014) and bronchial infection (p=0.006). Characteristics of patients with pneumonia are shown in Table 2. Regarding biomarkers levels, no significant differences were found when comparing patients according to previous antibiotic and corticosteroid therapy. Patients with congestive heart failure had higher levels of MR-proANP and MR-proADM (p<0.0001 and p=0.056, respectively). Regarding renal disease, levels of neopterin and MR-proANP were significantly higher (p=0.006, p=0.024). Neopterin levels were nearly significantly higher in patients with COPD as comorbidity (p=0.058). No significant differences were found when considering biomarkers levels and Charlson index classified as ≤1 and ≥2.
Levels of biomarkers in the three patient's group: pneumonia, ECOPD and bronchial infection without pneumonic infiltrate. Levels are expressed as median and IQR.
| Biomarker | Pneumonia | ECOPD | Bronchial infection |
| PCT (ng/mL) | 0.25 (0.09–2.04) | 0.09 (0.05–0.20) | 0.10 (0.06–0.25) |
| Neopterin (ng/mL) | 22.9 (13.83–40.1) | 19.38 (12.26–24.08) | 19.21 (12.18–32.57) |
| CRP (mg/L) | 116 (50–184.5) | 87 (20–154.5) | 55 (20.5–114.7) |
| MR-proANP (pmol/L) | 138.2 (63.6–272.8) | 109.9 (61.93–197.2) | 122.4 (56.08–251.07) |
| MR-proADM (nmol/L) | 1.1 (0.81–1.83) | 0.9 (0.74–1.08) | 0.87 (0.55–1.33) |
Characteristics of patients with a final diagnosis of pneumonia.
| Characteristics | N | % |
| Men | 59 | 69.4 |
| Women | 26 | 30.6 |
| Inpatient | 74 | 87 |
| Coexisting illnesses | ||
| Neoplasia | 30 | 35.3 |
| Liver disease | 7 | 8.2 |
| Congestive heart failure | 19 | 22.4 |
| Cerebrovascular disease | 9 | 10.6 |
| Renal disease | 11 | 12.9 |
| Chronic obstructive pulmonary disease | 23 | 27.1 |
| HIV | 7 | 8.2 |
| Previous antibiotic treatment | 16 | 18.8 |
| Previous corticosteroid therapy | 25 | 29.4 |
| Inhaled | 15 | 60 |
| Oral | 10 | 40 |
| Radiographic findings | ||
| Pleural effusion | 10 | 11.7 |
| Multilobar | 13 | 15.3 |
| Length hospitalization mean (SD) | 9.6 (10.27) | |
| ICU admission | 3 | 3.6 |
| Death | 8 | 9.4 |
Distribution of patients according to the severity pneumonia scores is shown in Table 3. MR-proANP and MR-proADM showed significant differences across PSI groups (p<0.001 and p=0.001, respectively) and higher levels were found in high risk (IV–V) in comparison to low risk (I–III) (p<0.001 and p<0.001, respectively) (Fig. 1). MR-proANP and MR-proADM levels also increased statistically with CURB-65 (p<0.001 and p<0.001, respectively) (Fig. 1). No statistical differences were found for PCT, CRP and neopterin.
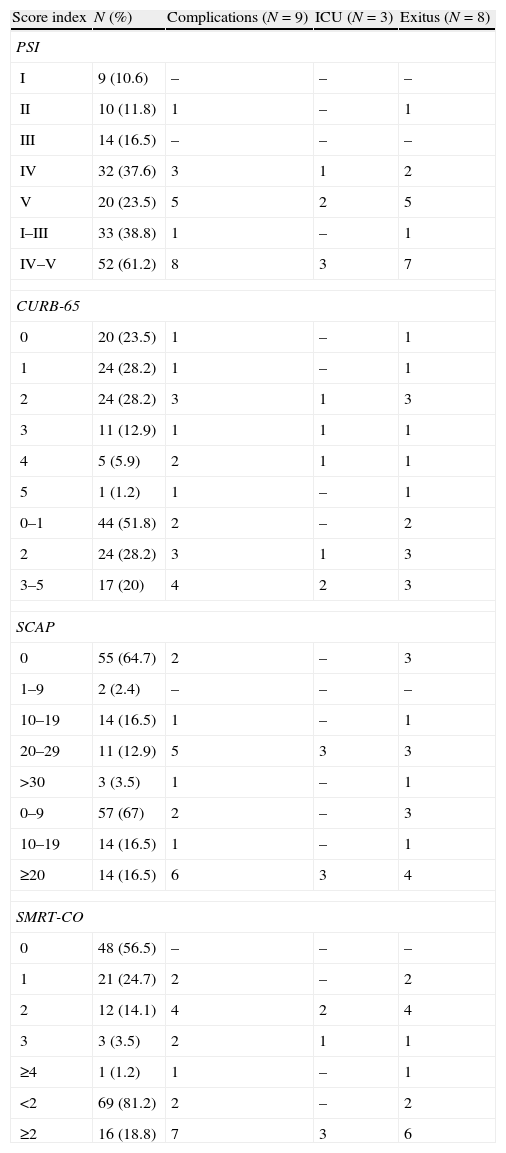
Distribution of patients according to the score results and the development of complications.
| Score index | N (%) | Complications (N=9) | ICU (N=3) | Exitus (N=8) |
| PSI | ||||
| I | 9 (10.6) | – | – | – |
| II | 10 (11.8) | 1 | – | 1 |
| III | 14 (16.5) | – | – | – |
| IV | 32 (37.6) | 3 | 1 | 2 |
| V | 20 (23.5) | 5 | 2 | 5 |
| I–III | 33 (38.8) | 1 | – | 1 |
| IV–V | 52 (61.2) | 8 | 3 | 7 |
| CURB-65 | ||||
| 0 | 20 (23.5) | 1 | – | 1 |
| 1 | 24 (28.2) | 1 | – | 1 |
| 2 | 24 (28.2) | 3 | 1 | 3 |
| 3 | 11 (12.9) | 1 | 1 | 1 |
| 4 | 5 (5.9) | 2 | 1 | 1 |
| 5 | 1 (1.2) | 1 | – | 1 |
| 0–1 | 44 (51.8) | 2 | – | 2 |
| 2 | 24 (28.2) | 3 | 1 | 3 |
| 3–5 | 17 (20) | 4 | 2 | 3 |
| SCAP | ||||
| 0 | 55 (64.7) | 2 | – | 3 |
| 1–9 | 2 (2.4) | – | – | – |
| 10–19 | 14 (16.5) | 1 | – | 1 |
| 20–29 | 11 (12.9) | 5 | 3 | 3 |
| >30 | 3 (3.5) | 1 | – | 1 |
| 0–9 | 57 (67) | 2 | – | 3 |
| 10–19 | 14 (16.5) | 1 | – | 1 |
| ≥20 | 14 (16.5) | 6 | 3 | 4 |
| SMRT-CO | ||||
| 0 | 48 (56.5) | – | – | – |
| 1 | 21 (24.7) | 2 | – | 2 |
| 2 | 12 (14.1) | 4 | 2 | 4 |
| 3 | 3 (3.5) | 2 | 1 | 1 |
| ≥4 | 1 (1.2) | 1 | – | 1 |
| <2 | 69 (81.2) | 2 | – | 2 |
| ≥2 | 16 (18.8) | 7 | 3 | 6 |
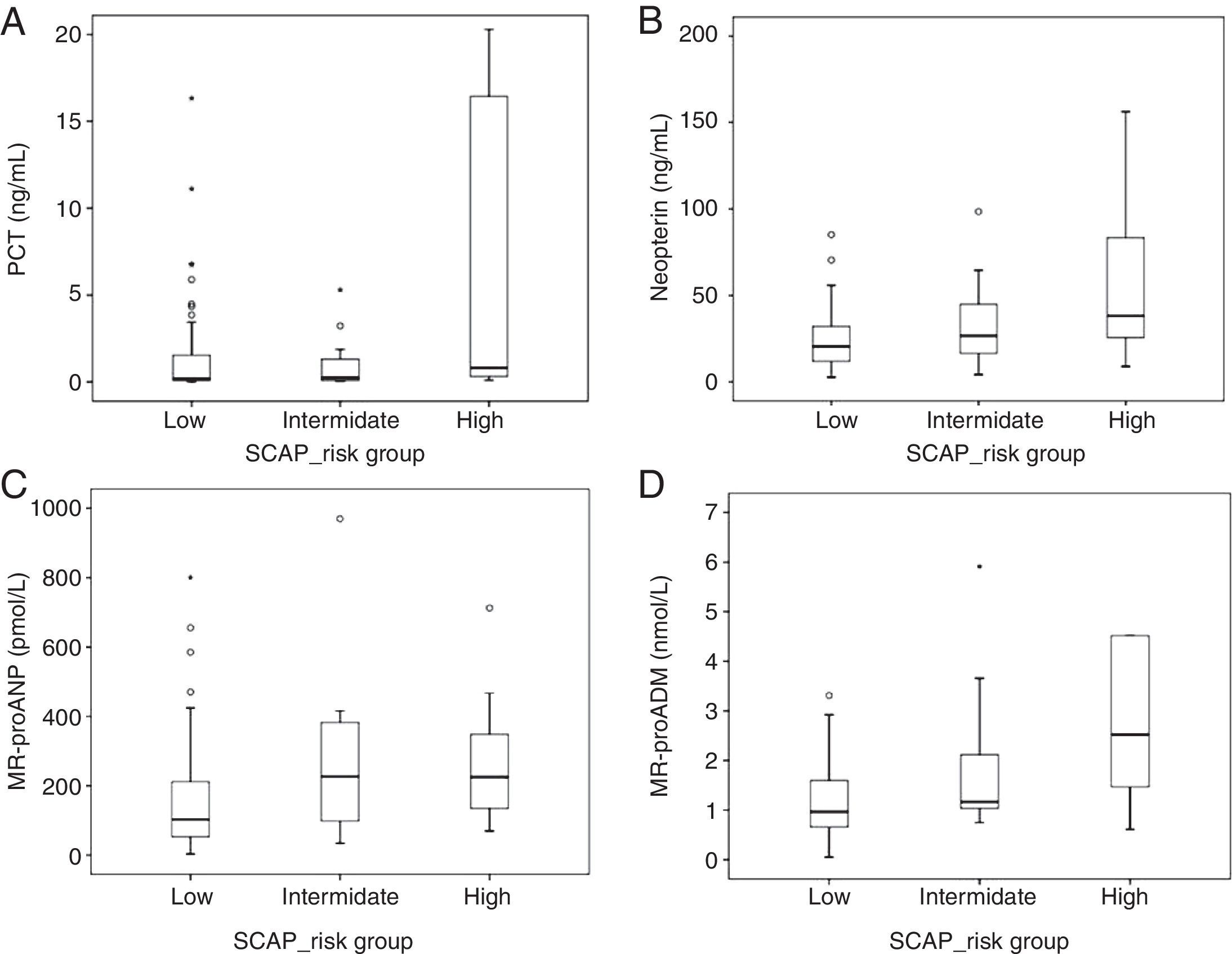
Regarding SCAP, thirty patients had SCAP criteria and showed higher levels of MR-proANP (p=0.002), MR-proADM (p=0.001), neopterin (p=0.011) and PCT (p=0.069) while for CRP levels no differences were found (p=0.549). When considering all pneumonia patients, regardless of SCAP criteria, MR-proANP (p=0.015) and MR-proADM (p=0.001) showed statistical differences across the five SCAP risk categories. SCAP score can also be classified according to low (0–9 points), intermediate (10–19) and high risk (≥20). PCT (p=0.010), neopterin (p=0.003), MR-proANP (p=0.010) and MR-proADM (<0.0001) showed higher levels in high risk group in comparison to low risk (Fig. 2). MR-proANP (p=0.049) and MR-proADM (p=0.051) also presented higher levels in intermediate risk in comparison to low risk.
The modified version SMRT-CO was available for all pneumonia patients. MR-proADM was the only biomarker that showed significant differences across score groups (p=0.051). Levels were statistically lower in patients with 0 (p=0.014) and 1 point (p=0.041) in comparison to patients with 3 points. Patients with ≥2 points had significantly higher levels of PCT (p=0.035) and MR-proADM (p=0.022), whereas for neopterin (p=0.060), CRP (p=0.147) and MR-proANP (p=0.694) no statistical differences were found (Fig. 3). In contrast, SMART-COP was only available for 28 patients. Fourteen patients had 0–2 points, 7 had 3–4 points, 6 had 5–6 points, and one patient had 8 points. None of the biomarkers showed statistical differences between score groups. However, neopterin levels were significantly higher in patients with ≥3 points in comparison to patients with <3 (p=0.021).
Nine patients developed complications related to the pneumonia and one patient died of a non-related cause which was cerebral lesion progression. Levels of all biomarkers, except CRP were higher in non-survivors (n=8) in comparison to survivors, although without statistical differences (data not shown). Patients developing pneumonia related complications showed statistically higher levels of neopterin (p=0.030) and MR-proADM (p=0.044). Levels of PCT (p=0.053), neopterin (p=0.003) and MR-proADM (p=0.001) were higher in patients admitted to ICU (n=3). The AUC (SE, 95% CI) to predict development of complications according to MR-proADM was 0.706 (0.11, 0.491–0.922). A cut-off of 0.95nmol/L had a sensitivity and specificity of 77.8% and 39.5%, respectively. A cut-off of 1.50nmol/L had a sensitivity and specificity of 66.7% and 65.8%, respectively. In Table 3 is also shown the distribution of patients according to the risk scores and the development of complications. It is of note that some patients were classified as low risk by all score indexes but still developed complications. Among these patients classified as low risk by severity scores, one of them was severely immunosuppressed with CD4<200 and died during the episode.
Microbiological and radiological findingsMicrobiological results for patients with pneumonia are shown in Table 4. PCT and MR-proADM showed significantly higher levels in cases of definite bacterial diagnosis in comparison to cases of probable bacterial origin (p=0.044 and p=0.028, respectively). If assembling cases of probable bacterial diagnosis and unknown origin, MR-proADM was the only biomarker that still showed a nearly significant difference when comparing to definite diagnosis group (p=0.059). A total of 6 blood cultures were positive: 3 for S. pneumoniae, 1 for Streptococcus mitis, 1 for Klebsiella pneumoniae, and 1 for Pseudomonas aeruginosa. Levels of MR-proADM were nearly significantly higher in patients with documented bacteremia in comparison to those with negative blood culture results (p=0.053). PCT (p=0.017) and MR-proADM (p=0.004) levels were also significantly higher when comparing patients with bacterial pneumonia with respect to other LRTI (including ECOPD and bronchial infections without pneumonic infiltrate) while no statistical differences were found for the other biomarkers.
Microorganisms isolated and PCT and MR-proADM levels according to the pneumonia etiology (median, interquartile range).
| Etiology | N | PCT (ng/mL) | MR-proADM (nmol/L) |
| Definite diagnosisa | 22 | 0.44 (0.09–3.97) | 1.50 (0.86–3.14) |
| Streptococcus pneumoniaeb | 16 | ||
| Viridans group streptococcic | 2 | ||
| Klebsiella pneumoniae | 1 | ||
| Pseudomonas aeruginosa | 1 | ||
| Pneumocystis jirovecii | 2 | ||
| Probable diagnosisd | 12 | 0.13 (0.06–0.36) | 0.90 (0.61–1.37) |
| S. pneumoniae | 6 | ||
| Haemophilus influenzae | 3 | ||
| Moraxella catarrhalis | 1 | ||
| Other gram negative | 2 | ||
| Unknown origin | 51 | 0.28 (0.09–1.87) | 1.06 (0.84–1.76) |
In 13 cases there was multilobar involvement and neopterin was the only biomarker that showed significantly higher levels (p=0.011). Although there were no statistical differences, all biomarkers were higher in patients with pleural effusion.
DiscussionThe correct clinical management of patients with LRTI in the ED is still a challenge because of the difficulties in clinical decisions regarding antibiotic treatment and site of care. Diagnostic mistakes in the ED are more frequent in patients presenting with fever and respiratory symptoms.33 In this setting, biomarkers could act as a complementary tool, together with clinical and microbiological criteria, for the right identification of patients with bacterial pneumonia and patients at risk of developing complications.13,34
In one hand, for severity assessment, biomarkers measurement can predict disease severity in CAP patients, aiming to complement the available risk scores.12 In our study, the correlation of MR-proANP and MR-proADM levels with validated risk scores: PSI and CURB-65 has been confirmed. In fact, several studies have shown that the combination of score risks, mainly PSI and CURB-65, with a biomarker measurement can improve the prediction of outcome.21,24,35,36 In our previous experience, we already demonstrated that MR-proANP correlates significantly with both rules and that this correlation is not affected by the presence of cardiac and renal comorbidities.18 Regarding MR-proADM, we confirm previous findings that demonstrate that MR-proADM admission levels correlate with a similar accuracy as these scores.21,23,25,36,37
To our knowledge this is the first study that has explored the correlation of biomarkers with severity scores: SCAP and SMART-COP. MR-proANP and MR-proADM showed a strong correlation with SCAP, since higher levels were found in patients in high and intermediate risk in comparison to patients classified as low risk. Surprisingly, two patients who developed respiratory failure/shock and died did not have SCAP criteria. One HIV patient had P. jirovecii pneumonia and died 50 days later and MR-proADM levels were of 0.59nmol/L. The other one was a severely immunosuppressed patient with megaloblastic anemia who died because of respiratory failure after 12 days and whose MR-proADM levels were of 0.95nmol/L. There is another patient who did not have SCAP criteria but died of a cause not related to pneumonia, whose MR-proADM and MR-proANP levels were 0.96nmol/L and 208.30pmol/L, respectively. Regarding SMART-COP, only neopterin showed significantly higher levels in patients with ≥3 points. However, when considering its simplified version SMRT-CO, that was available for all patients, PCT and MR-proADM levels were higher in patients classified as moderate-high risk of needing IRVS in comparison to patients with very low/low risk.
Given that severity scores were designed and validated in immunocompetent population, when applying them directly to immunosuppressed patients it is possible that some severity score might misclassify them. In addition immunosuppressed patients are also likely to have a diminished inflammatory response, and therefore influence the relationship between severity score and biomarkers levels.
Two different cut-offs for the prediction of mortality in pneumonia patients have been proposed for MR-proADM: 1.8nmol/L and 1.3nmol/L.36,37 In our experience, we chose a range of cut-off: 0.95nmol/L and 1.50nmol/L that corresponded to higher sensitivity and specificity.
On the other hand, the identification of definite bacterial origin has direct implications in antibiotic treatment decisions. Unfortunately, microbiological diagnosis of pneumonia is only achieved in about 50% of the cases.27 Our results show that PCT and MR-proADM levels are significantly higher in cases of definite bacterial diagnosis in comparison to pneumonia of probable bacterial or unknown etiology. This reinforces the fact that both biomarkers have a strong relationship with bacterial etiology. In fact, values were higher in patients with bacterial pneumonia with respect to other LRTI. Recent clinical studies have shown that PCT38,39 and MR-proADM36,40 help in the prediction of bacteremia. This is of importance because the presence of bacteremia is associated with mortality.41 In fact, both biomarkers belong to the calcitonin gene family and are widely and extensively synthesized during severe infections.42 PCT has been broadly evaluated in different clinical settings.10,39,43–45 ADM has pleiotropic effects and its role as antimicrobial agent might explain why its levels increase during sepsis46 being also likely responsible for the hypotension characteristic of septic shock.14
This study has some limitations. Firstly, study inclusion was limited to patients likely to have a severe condition admitted to an ED of a tertiary hospital within a two month period, so patients with milder symptoms were excluded. Secondly, although in our experience no significant differences were found, it is not unlikely that some biomarkers might be influenced by previous antibiotic and corticosteroid treatment. Thirdly, ICU admission criteria can differ between hospitals. And finally, that the number of patients analyzed is small and so, it is not possible to draw robust conclusions.
In summary, in patients with final diagnosis of pneumonia, MR-proADM and MR-proANP levels correlate significantly with validated PSI and CURB-65 index. MR-proADM has also shown higher levels in high risk groups in comparison to low risk groups, when assessing SCAP and modified SMART-COP. MR-proANP and PCT have also shown a correlation with SCAP and SMRT-CO. This is the first study evaluating the relationship between inflammatory and cardiovascular biomarkers and SCAP and SMART-COP indexes and although the number of patients is low it provides preliminary useful and important data that have to be confirmed in further studies.
Conflicts of interestNo company had a role in the design or conducting of the study, collection, management, or interpretation of the data, preparation, review, or approval of this manuscript. The authors have no conflict of interest, including specific financial interests or relationships or affiliations to the subject matter or materials discussed in the manuscript.
The company BRAHMS Thermo Fisher has supplied the necessary kits of PCT and MR-proADM. This study was supported by a grant of Fundació Catalana de Pneumologia (FUCAP). We thank Microbiology and Hematology Laboratory technicians of the Hospital Universitari Germans Trias i Pujol for the help in sample collection and Oriol Martos for technical assistance. J. Domínguez is a researcher of the “Miguel Servet” programme of the Instituto de Salud Carlos III (Spain).