The National AIDS Plan and the Spanish AIDS study group (GESIDA) proposes “preferred regimens” (PR) of antiretroviral treatment (ART) as initial therapy in HIV-infected patients. In 2013, the recommended regimens were all triple therapy regimens. The Gardel Study assessed the efficacy of a dual therapy (DT) combination of lopinavir/ritonavir (LPV/r) plus lamivudine (3TC). Our objective is to evaluate the GESIDA PR and the DT regimen LPV/r+3TC cost/efficacy ratios.
MethodsDecision tree models were built. Efficacy: probability of having viral load <50 copies/mL at week 48. ART regime cost: costs of ART, adverse effects, and drug resistance tests during the first 48 weeks.
ResultsCost/efficacy ratios varied between 5,817 and 13,930 euros per responder at 48 weeks, for the DT of LPV/r+3TC and tenofovir DF/emtricitabine+raltegravir, respectively.
ConclusionsTaking into account the official Spanish prices of ART, the most efficient regimen was DT of LPV/r+3TC, followed by the triple therapy with non-nucleoside containing regimens.
El Plan Nacional sobre el Sida y el grupo de estudio del SIDA-SEIMC (GESIDA) propone «regímenes preferentes» (RP) de tratamiento antirretroviral (TAR) como terapia inicial en pacientes infectados por VIH. Todos los regímenes recomendados en el año 2013 eran de terapia triple. El Estudio Gardel evaluó la eficacia de una doble terapia (DT) que combina lopinavir/ritonavir (LPV/r) más lamivudina (3TC). Nuestro objetivo es evaluar los ratios de coste/eficacia de los RP de GESIDA y el régimen de DT LPV/r+3TC.
MétodosConstrucción de árboles de decisión como modelos de evaluación económica. Eficacia: probabilidad de tener una carga viral <50 copias/ml en la semana 48. Coste del régimen de TAR: coste del TAR, efectos adversos y tests de resistencia durante las primeras 48 semanas.
ResultadosLos ratios de coste/eficacia variaron entre 5.817€ y 13.930€ por respondedor a las 48 semanas, para la DT LPV/r+3TC y tenofovir DF/emtricitabina+raltegravir, respectivamente.
ConclusionesCon los precios oficiales españoles de TAR, el régimen más eficiente fue la DT con LPV/r+3TC, seguida de la terapia triple con regímenes que no contienen nucleósidos.
The National AIDS Plan and the Spanish AIDS study group (GESIDA) panel of experts propose, every year, “preferred regimens” (PR) of antiretroviral treatment (ART) as initial therapy for HIV infected patients. The PR for the year 2013 were all triple therapy regimens.1 After the publication of the 2013 GESIDA preferred regimens, the GARDEL (Global AntiRetroviral Design Encompassing Lopinavir (LPV)/ritonavir (r) and Lamivudine (3TC) vs LPV/r based standard therapy) Study was published.2 The GARDEL trial assessed the efficacy and safety of a dual therapy (DT) combination of LPV/r 400/100mg+3TC 150mg, both twice daily compared to triple therapy regimens of LPV/r 400/100mg twice daily plus 3TC or emtricitabine (FTC) plus one other, investigator selected nucleos(t)ide reverse transcriptase inhibitor. The objective of this study is to evaluate the costs and efficiency of initiating treatment with the GESIDA PR and the DT of LPV/r+3TC.
MethodsDesign: Economic assessment of the costs and efficiency (cost/efficacy) by building decision trees with deterministic sensitivity analysis. The decision trees for calculating the costs, efficacy, and efficiency of the 2013 PR have been previously published.3 A new decision tree was built for the DT following the same methodology. The design, methods for building the trees, sources of data, definition of efficacy, costs and efficiency, use of resources, and sensitivity analysis have been the same as those used previously for the 2013 PR.3 In summary, such methods have been the following.
Perspective: The Payer (Spanish National Health System) perspective was applied. The following differential direct costs were considered: (1) ART (Laboratory sale price+4% VAT – 7.5% obligatory legal reduction). In the case of DT, for 3TC, the generic price (65.27 euros: Laboratory sale price+4% VAT) was considered, resulting in a cost of 5,041 euros for 48 weeks; (2) Adverse events (AE) management (drug treatment, emergency room visits, additional visits to the HIV specialist, visits to other specialists, diagnostic tests, and hospital admissions). Each unitary cost was calculated as the mean of the official prices of the Autonomous Communities (regions) Health Services; and (3) Genotypic study of drug resistance and HLA B*5701 testing.
Time horizon: 48 weeks.
Cost of initiating a regimen: Cost of ART and all the consequences (adverse effects, changes of ART regimen and drug resistance tests) incurred in 48 weeks due to the decision of initiating ART with that regimen. The substitution regimens for DT depending of the cause were decided by two of the authors (JMG and JRA): Tenofovir DF (TDF)/FTC+LPV/r for viral failure and pregnancy, TDF/FTC/efavirenz (EFV) for adverse effect, TDF/FTC+darunavir (DRV)/r for lack of adherence, and DT for lost to follow-up and others causes.
Efficacy: Quotient of the number of patients with undetectable viral load (<50 copies/mL) at week 48 post-ART (i.e., responders) (numerator) and the number of patients initiated on ART (denominator). It was estimated based on an intention-to-treat analysis of the exposed (“Intent-to-treat exposed” [ITT-E]), “missing or non-completer=failure”).
Efficiency: Defined in terms of cost/efficacy and calculated for each regimen as the quotient of the cost of initiating treatment with that regimen (numerator) and efficacy (denominator). It represents the cost of achieving one responder by week 48.
Sources of information: Clinical trials (CTs): Data on efficacy, AE and withdrawals. Data of CTs included in a previous study3 and the GARDEL Study.2 The expert opinion was used when scientific evidence was not available (substitution regimens and resources used in AE management).
Uncertainty management: Deterministic sensitivity analysis, building scenarios with 95% confidence intervals for efficacy and AE probability, and ±15% for costs.
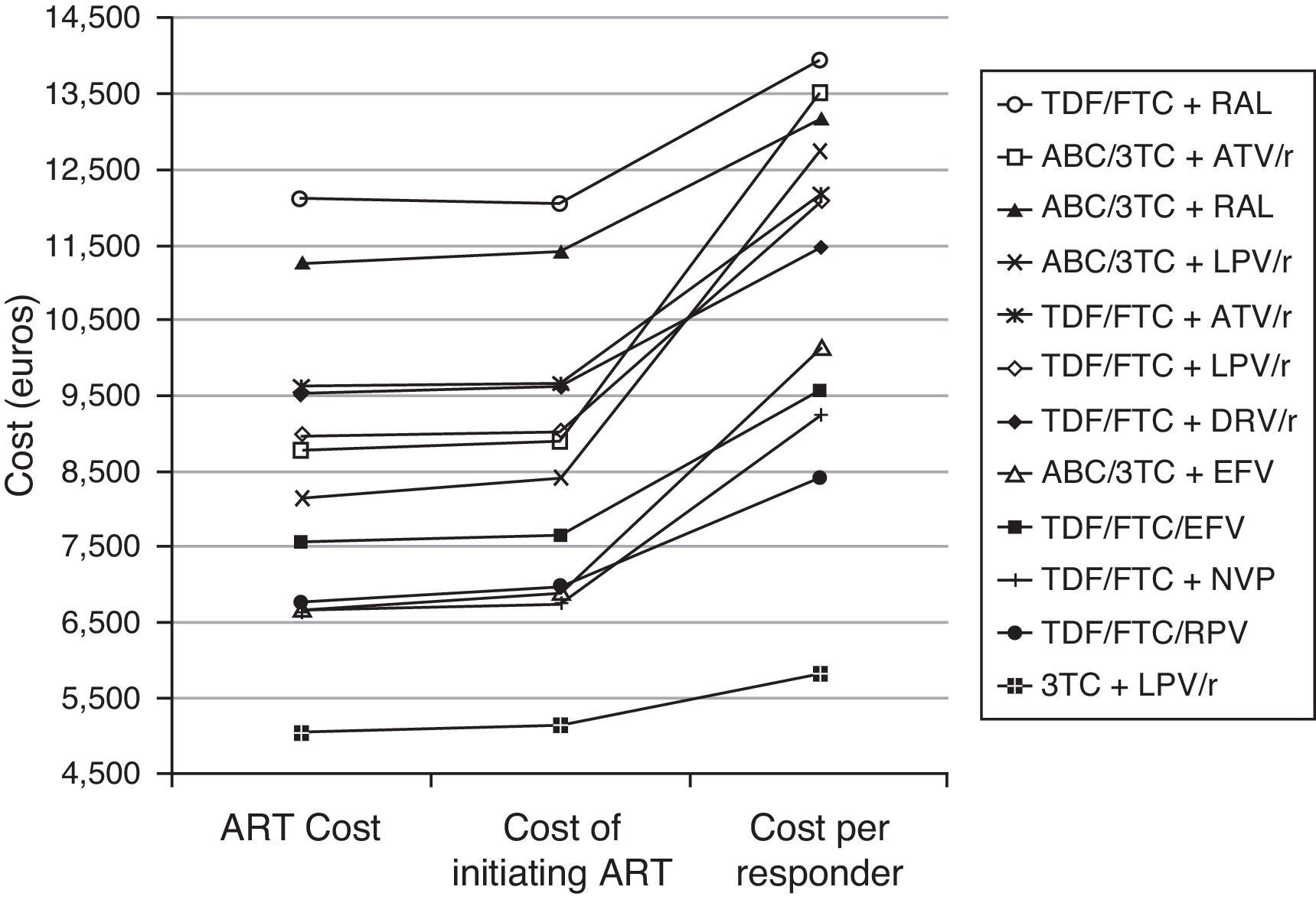
ResultsIn the base case scenario, the cost of initiating treatment ranges from 5,138 euros for DT of LPV/r+3TC to 12,059 euros for TDF/FTC+raltegravir (RAL). The efficacy ranged between 0.66 for abacavir (ABC)/3TC+LPV/r and ABC/3TC+atazanavir (ATV)/r, and 0.88 for DT of LPV/r+3TC. The efficiency, in terms of cost/efficacy, varied between 5,817 and 13,930 euros per responder at 48 weeks, for DT of LPV/r+3TC and TDF/FTC+RAL respectively. Moreover the DT regimen was the most efficient regimen in the most favorable (5,503 euros per responder) and most unfavorable (6,169 euros per responder) scenarios (Table 1 and Fig. 1).
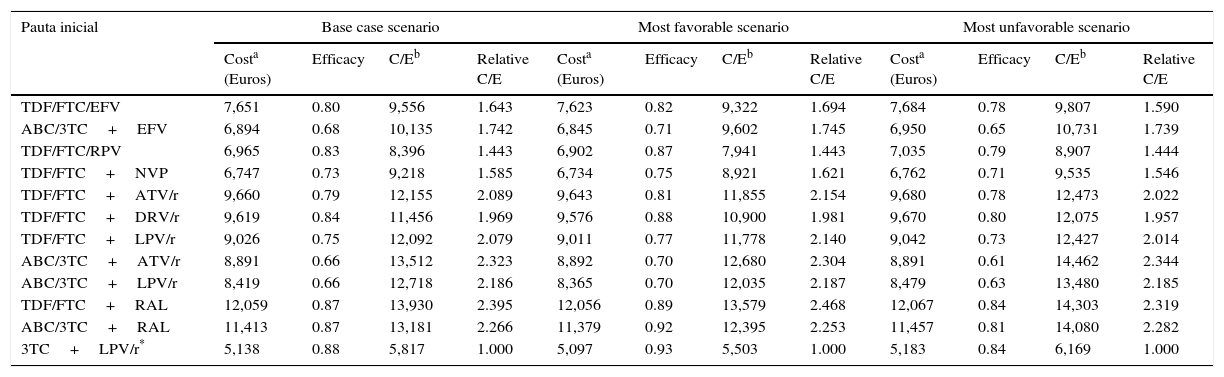
Cost, efficacy, efficiency (cost/efficacy) and relative efficiency of initiating treatment with each regimen (using the regimen 3TC+LPV/r as the reference). Sensitivity analysis.
| Pauta inicial | Base case scenario | Most favorable scenario | Most unfavorable scenario | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Costa (Euros) | Efficacy | C/Eb | Relative C/E | Costa (Euros) | Efficacy | C/Eb | Relative C/E | Costa (Euros) | Efficacy | C/Eb | Relative C/E | |
| TDF/FTC/EFV | 7,651 | 0.80 | 9,556 | 1.643 | 7,623 | 0.82 | 9,322 | 1.694 | 7,684 | 0.78 | 9,807 | 1.590 |
| ABC/3TC+EFV | 6,894 | 0.68 | 10,135 | 1.742 | 6,845 | 0.71 | 9,602 | 1.745 | 6,950 | 0.65 | 10,731 | 1.739 |
| TDF/FTC/RPV | 6,965 | 0.83 | 8,396 | 1.443 | 6,902 | 0.87 | 7,941 | 1.443 | 7,035 | 0.79 | 8,907 | 1.444 |
| TDF/FTC+NVP | 6,747 | 0.73 | 9,218 | 1.585 | 6,734 | 0.75 | 8,921 | 1.621 | 6,762 | 0.71 | 9,535 | 1.546 |
| TDF/FTC+ATV/r | 9,660 | 0.79 | 12,155 | 2.089 | 9,643 | 0.81 | 11,855 | 2.154 | 9,680 | 0.78 | 12,473 | 2.022 |
| TDF/FTC+DRV/r | 9,619 | 0.84 | 11,456 | 1.969 | 9,576 | 0.88 | 10,900 | 1.981 | 9,670 | 0.80 | 12,075 | 1.957 |
| TDF/FTC+LPV/r | 9,026 | 0.75 | 12,092 | 2.079 | 9,011 | 0.77 | 11,778 | 2.140 | 9,042 | 0.73 | 12,427 | 2.014 |
| ABC/3TC+ATV/r | 8,891 | 0.66 | 13,512 | 2.323 | 8,892 | 0.70 | 12,680 | 2.304 | 8,891 | 0.61 | 14,462 | 2.344 |
| ABC/3TC+LPV/r | 8,419 | 0.66 | 12,718 | 2.186 | 8,365 | 0.70 | 12,035 | 2.187 | 8,479 | 0.63 | 13,480 | 2.185 |
| TDF/FTC+RAL | 12,059 | 0.87 | 13,930 | 2.395 | 12,056 | 0.89 | 13,579 | 2.468 | 12,067 | 0.84 | 14,303 | 2.319 |
| ABC/3TC+RAL | 11,413 | 0.87 | 13,181 | 2.266 | 11,379 | 0.92 | 12,395 | 2.253 | 11,457 | 0.81 | 14,080 | 2.282 |
| 3TC+LPV/r* | 5,138 | 0.88 | 5,817 | 1.000 | 5,097 | 0.93 | 5,503 | 1.000 | 5,183 | 0.84 | 6,169 | 1.000 |
ABC, abacavir; ATV, atazanavir; COBI, cobicistat; DRV, darunavir; EFV, efavirenz; EVG, elvitegravir; FTC, emtricitabine; LPV, lopinavir; NVP, nevirapine; /r, ritonavir-boosted; RAL, raltegravir; RPV, rilpivirine; TDF, tenofovir DF; 3TC, lamivudine.
Cost and cost per responder (efficiency) of the GESIDA 2013 preferred regimens and dual therapy (3TC+LPV/r). Base case scenario. ART Cost: Drug costs for each regimen for 48 weeks (laboratory sale price (LSP)+4% VAT – 7.5% reduction). Cost of initiating ART: cost of initiating a regimen including all potential consequences of initiating ART with that regimen (Adverse effects and changes to other regimens) that may occur within 48 weeks. Cost per Responder: Cost of achieving one responder (<50 copies of RNA of HIV per mL of plasma) by week 48 for the National Health Service. This is calculated as the cost of initiating an ART divided by its efficacy. ABC, abacavir; ATV, atazanavir; DRV, darunavir; EFV, efavirenz; FTC, emtricitabine; LPV, lopinavir; NVP, nevirapine; /r, ritonavir-boosted; RAL, raltegravir; RPV, rilpivirine; TDF, tenofovir DF; 3TC, lamivudine.
Since local cost of a specific hospital may be different to the costs used in the model, a software application was designed for allowing the calculation of ART costs, regimen initiation costs, efficiency (cost/efficacy), and relative efficiency of initiating treatment with the different regimens at each individual hospital setting. The application is available free of charge at: https://www.dropbox.com/s/875w8ye95j640ci/Aplicacion-TARV-VIH-2013-TerapiaDoble.exe?dl=0.
DiscussionAccording to the findings of this analysis, considering the ART official Spanish prices, the most efficient regimen was LPV/r+3TC, followed by triple therapy with non-nucleoside containing regimens recommended in 2013 by the Spanish GESIDA expert panel. This finding should be interpreted carefully taking into account some limitations. The specific limitations affecting the GARDEL trial and the cost/efficacy analysis performed for the GESIDA recommended regimens are described in their respective articles.2,3 But some limitations should be mentioned. For example, the analyses presented here are based on clinical trials performed in different countries, in different years (published between 2006 and 2014), with different inclusion and exclusion criteria, and even with different presentations for the same drug in some regimens. Thus, the results may have differed if all regimens had been administered in similar populations and years. Actually, recent studies include lower percentages of patients with poor prognosis, i.e., those with low CD4 counts (<100/200 cells/μL) and elevated plasma viral load (>100,000 copies/mL). This leads to results with higher efficacy in recent studies than those reported in previous studies and may offer an advantage to drugs assessed recently for the first time. Also, when more than one clinical trial assessed the same regimen, a metanalysis could not be performed because of the absence of a common comparator. Similarly, because of lack of scientific evidence, the substitution regimens used when the initial regimen was suspended were estimated based on experts’ opinion. Although the substitution regimens were stablished with agreement among all the authors, some doctors could have preferences for other substitution regimens. Another limitation would be that these findings are applicable only to Spain and taking into account the Spanish official drug prices. Finally the DT regimen was compared with the GESIDA PR of 2013, but the list of PR or alternatives for 20144 and most likely for 2015 will be very similar.
The strengths of this study include the use of the best scientific available evidence and a sensitivity analyses for managing the underlining uncertainty in costs and outcomes. Further, the models use efficacy estimators, with universal validity, which, added to the fact that the methodology is applicable to any environment, would make the results valid in other contexts as long as local costs could be entered into the models. For this reason, in order to facilitate the use of this methodology in centers or countries with different ART- or HIV management-related costs or to take into account the potential future use of generic drugs,5 a software application was developed and made available free of charge (see methods). This application allows the calculations of ART costs, initiating ART costs, efficiency (cost/efficacy), and the relative efficiency of initiating treatment with the different regimens based on local costs.
Since the publication of the Gardel study2 the DT regimen (LPV/r+3TC) has been recommended as an alternative for initial ART (without restrictions of CD4+ cell counts or plasma viral load) by international guidelines like EACS6 or IAS-USA.7 Conversely, several DT regimens tested for initial therapy have failed8 or can only be recommended with restrictions.9
In conclusion, the DT regimen (LPV/r+3TC) is one of the most efficient alternatives for initial ART and can be recommended without restrictions of CD4+ cell counts or plasma viral load. Whether these outcomes can be extrapolated to other potential DT regimens like darunavir/r+3TC or Dolutegravir+3TC remain to be tested.
Conflict of interestJosep M. Gatell has received honoraria for speaking or participating in Advisory Boards and/or research grants from BMS, MSD, Tobira, Gilead, BI, Janssen, ViiV and Abbvie.
José Ramón Arribas receives advisory fees, speaker's fees, or grant support from Viiv, Tibotec, Janssen, Abbvie, Bristol-Myers Squibb, Gilead Sciences, Merck Inc, and Tobira.
Pablo Lázaro and Antonio Javier Blasco have no potential conflicts of interest related to this study.
The authors acknowledge the funding support from the Fundación Máximo Soriano Jiménez and Red Española de Investigación en SIDA (RIS).