It has been reported that microbiological diagnosis of Cutibacterium spp. infection requires a prolonged incubation time (up to 14 days). We present our experience with regard to incubation time for detection of Cutibacterium spp. in orthopaedic samples over a 10-year period.
MethodsOne hundred and nineteen samples were included in this retrospective study. Fifty-three were implants (having previously undergone sonication), 64 were periprosthetic tissue biopsies and two were synovial fluids. Atkins's criteria were used for interpreting the isolates. Quantification and number of days until a culture became positive for Cutibacterium spp. were evaluated.
ResultsThe median number of days to detection of a clinically significant isolate and a contaminant was 4 days. No clinically significant isolates grew after day eight.
ConclusionMost clinically significant isolates of Cutibacterium spp. are detected in the first 7 days of incubation, although a recommendation of prolonged incubation (up to 14 days) appears to be necessary for detecting other organisms.
Se ha reportado que el diagnóstico microbiológico de las infecciones por Cutibacterium spp. requiere un tiempo de incubación prolongado (hasta 14 días). Presentamos nuestra experiencia al respecto en muestras ortopédicas durante un período de 10 años.
MétodosSe incluyeron en este estudio retrospectivo 119 muestras de las que 53 fueron implantes (previa sonicación), 64 biopsias de tejido periprotésico y dos líquidos sinoviales. Para la interpretación se siguieron los criterios de Atkins. Se evaluó la cuantificación y el número de días hasta que el cultivo fue positivo para Cutibacterium spp.
ResultadosLa mediana del número de días para detectar un aislado clínicamente significativo y un contaminante fue de cuatro días. Ningún aislado clínicamente relevante creció después del día ocho.
ConclusiónLa mayoría de aislados clínicamente significativos de Cutibacterium spp. se detectan durante los siete primeros días de incubación, sin embargo, parece necesaria una incubación de hasta 14 días para la detección de otros microorganismos.
Cutibacterium acnes is a Gram-positive anaerobic rod that is part of the human skin microbiota.1 It has been described as an opportunistic pathogen, especially associated with different indwelling biomaterials, such as breast implant, cardiac devices or orthopaedic prostheses, as well as with some surgical procedures. Its ability to form biofilms is considered one of the most important pathogenic factors associated to these type of infections,2 and as long as it is an infrequent pathogen, its treatment is not well defined in some scenarios.
Diagnosis of infections caused by this organism has been claimed to need a prolonged incubation time (up to 14 days) because the slow growing rate of this species.1,3–6 However, there are other reports suggesting that a shorter incubation time is able to detect these isolates when they are clinically significant.7–9 We retrospectively analyse the incubation time needed to detect C. acnes growth in implant-associated samples (including implants and periprosthetic tissues) over a 10-year period.
Materials and methodsRecords from the Clinical Microbiology Department were reviewed for samples with isolation of Cutibacterium spp. between June 2009 and June 2019. Isolates from orthopaedic implant-associated infections samples were selected for analysis. Only pure cultures were selected. During this period, the management of the samples was maintained without changes.7 Knee and hip samples from patients with clinical suspicion of infection according to the Infectious Diseases Society of America (IDSA) criteria and from all shoulder or elbow implants were incubated up to 14 days. Isolates were considered significant or not according to the Atkins’ criteria10 and the clinical and analytical data of the patients (IDSA criteria11). Specifically, anaerobic culture samples were inoculated onto Schaedler-5% sheep blood agar plates (BioMérieux, Marcy l’Étoile, France) and incubated in anaerobic jars that were maintained closed for the first 48h, and then examined daily until they were discharged. Clinical isolates were identified at species level using RAPID-ID32A (Biomérieux, Marcy l’Étoile, France) and MALDI-TOF (Vitek MS, BioMérieux, Marcy l’Étoile, France). Quantification of the sonicated implant samples was performed as previously published.7 All other samples were evaluated using a semiquantitative approach as abundant growth (3 streaks), moderate growth (2 streaks) and scanty growth (1 streak). The Laboratory Internal Software (SGLAC, Spain) allowed to perform the evaluation of the number of days between inoculation and growth detection through the internal data base. The study was approved by the Ethics in Research Committee of our hospital.
Statistical analysis was performed using the Stata Statistical Software: Release 11 (StataCorp, College Station, Texas, USA). Data were evaluated using a one-sided Wilcoxon nonparametric test was applied to compare two groups, and Shapiro–Wilk parametric test to verify normal distribution. Statistical significance was set at p-values ≤0.05. Comparisons were performed only between quantitative cultures (sonicated implants) and another one between semiquantitative cultures (other samples), without mixing both types of samples.
ResultsDuring the study period, 119 culture samples (62 patients) from orthopaedic implants were positive for Cutibacterium spp. All strains were identified as Cutibacterium acnes, except one of them that was identified as Cutibacterium avidum.
Fifty-three samples were isolated from implants, 64 from tissue biopsies and 2 from synovial fluid. Eighty-three samples from 29 patients were isolated from patients with implant-associated infections and were considered clinically significant, while 35 isolates (33 patients) were considered contaminants. Twenty four samples came from patients with osteosynthesis (10 clinically significant isolates), 41 from patients with shoulder prosthesis (36 clinically relevant), 32 with hip prosthesis (26 clinically relevant), 20 with knee prosthesis (11 clinically significant), and 2 with elbow prosthesis (both non-significant ones). Over the study period 723 prosthetic joint infections (PJI) were diagnosed (Cutibacterium spp. was the cause of 3.95% of all infections).
Implant isolates were quantified, and the median (interquartile range) CFU/ml was 1000 (400–50,000) CFU/ml for clinically significant samples (range 100–100,000CFU/ml) and 750 (200–10,000)CFU/ml for contaminants (range 100–50,000CFU/ml) (p=0.0686, non-significant for Wilcoxon test). Regarding the tissue samples, C. acnes isolation had clinical significance in 13 (high or moderate growth, in contrast with contaminants: scarce growth or moderate in one case).
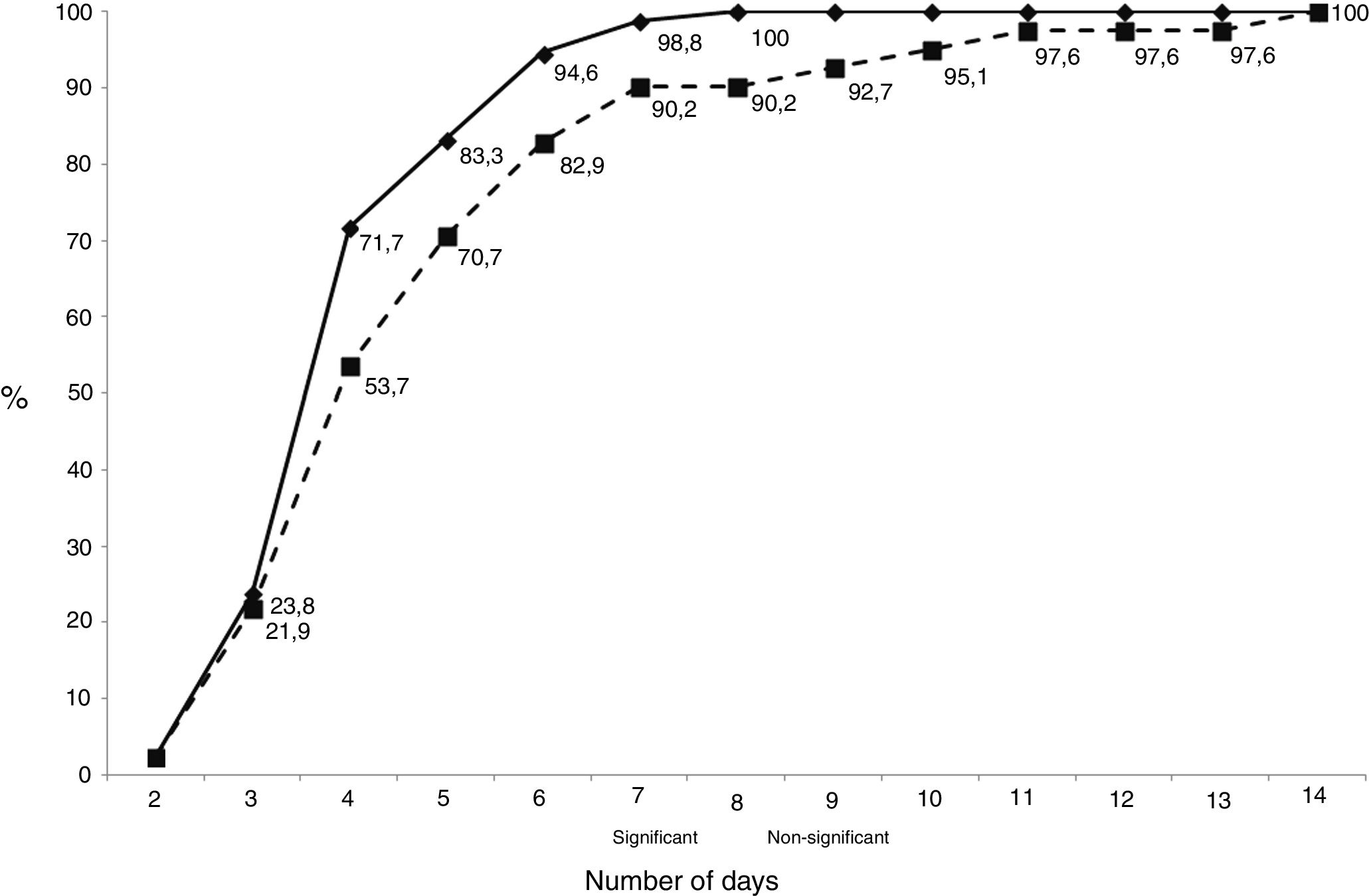
The median (interquartile range) number of days necessary to detect an isolate was 4, same for clinically significant isolates (3–5) than for contaminants (3.75–6). The cumulative number of days necessary to detect growth in the cultures is shown in Fig. 1. Only 1 sample gave a positive result after day 7 (day 8), and this patient had 2 samples that grew at days 4 and 5 (in fact, diagnosis was performed at day 4).
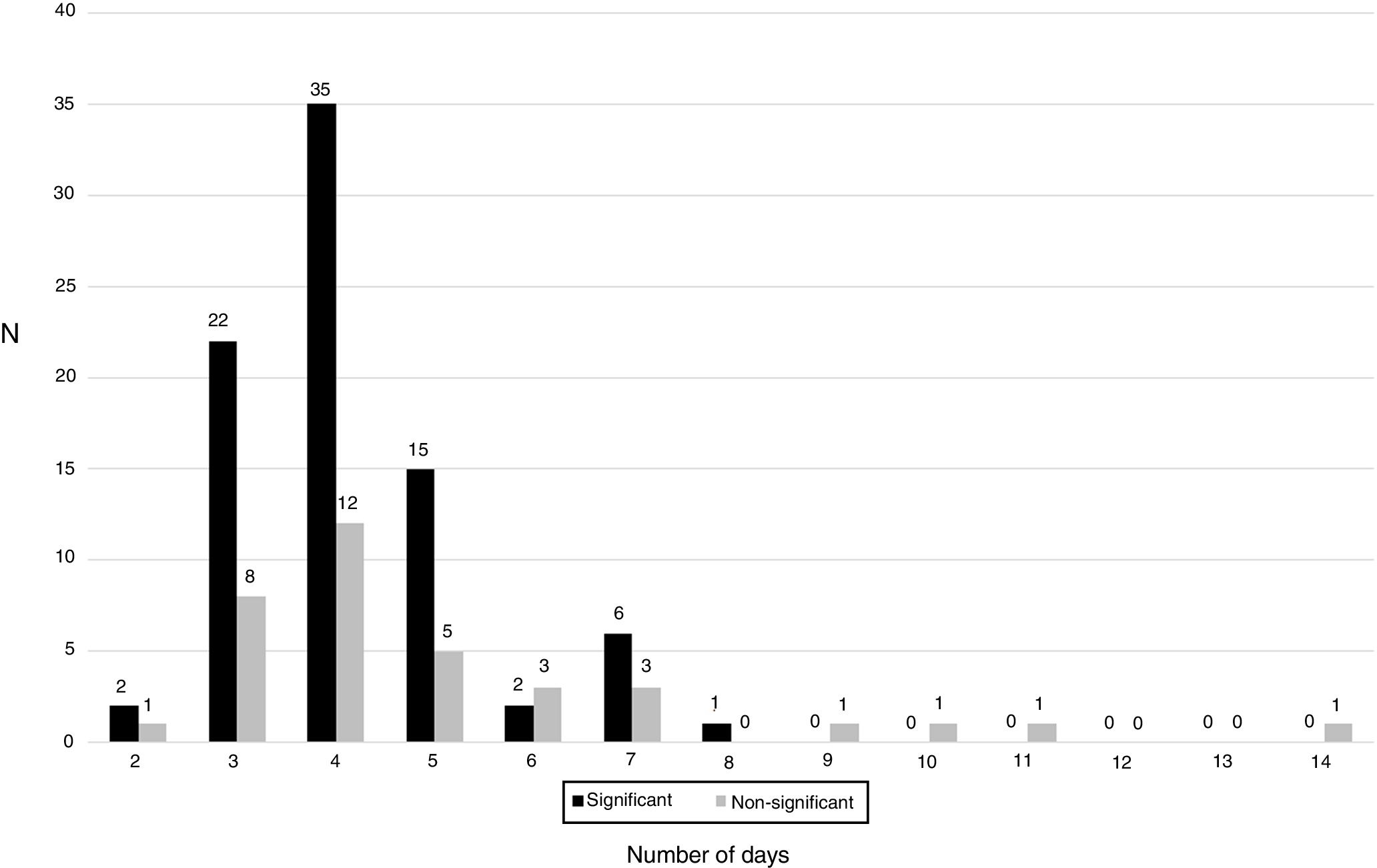
As shown in Fig. 2, the distribution of clinically significant isolates followed a Gaussian pattern with a mean±deviation standard of 4.2±1.2 days (p=0.1661 for Shapiro–Wilk test). Almost all of them grew before day 8 (98.8%, 83/84). No clinically relevant isolate was detected after day 7. The distribution of contaminants did not follow a normal pattern (p=0.0061 for Shapiro–Wilk test).
DiscussionCutibacterium spp. have been claimed to be difficult to recover from prosthetic joint infections, as they are most often related to positive intraoperative cultures and late chronic infections, following Tsukayama scheme to classify prosthetic joint infections.12 In the study of Bossard et al. a prolonged incubation time of 10 days is recommended,3 as well as in other studies1,4,6,13,14 due to its low microbiological yield. This can be related to the ability of Cutibacterium spp. to adhere to the implant surface and form biofilms, requiring the use of sonication techniques7 and increased incubation time in enriched culture media.5,13 However, in our series all clinically relevant isolates, and even most contaminant strains, grew in the first 7 days of incubation, as previously published.5,7,15 Some potential reasons for these discrepancies could be the selection of the medium used: an enriched medium could have better recoveries.8 In our study, we used Schaedler agar (with casein hydrolysate, L-cystine, dextrose and dipotassium hydrogen phosphate) plus 5% sheep blood. The use of this enriched medium probably allowed to obtain growth in less than 7 days. This is in accordance to the study of Jeverica et al. where the time to detection of C. acnes isolates was 48–72h after inoculation in Schaedler agar. Other studies included liquid media like thioglycolate broth.1,3,13,15 The problem of just using this medium is the great probability of detecting a contaminant. In fact, C. acnes is a microorganism with a very homogeneous biotype and antibiotype, so molecular techniques are necessary in order to differentiate strains. Moreover it has been described that genotypic differences among C. acnes isolates using multilocus sequence typing (MLST), are related to different clinical manifestations of C. acnes PJI and may help to differentiate between infection and contamination.16 This must be taken into account for a proper use of the Atkins criteria.10 However, it is possible for some patients, especially if antibiotics are administered before surgery, to have true positive cultures from the liquid media. Probably, in these patients, interpretation of liquid cultures must be performed cautiously. In our series, the percentage of culture-negative PJI was lower than 5%,17 so we think that the number of Cutibacterium spp. infections missed must be low, unlike other series.18
Several authors have proposed to prolong the incubation time for at least 14 days,5,6,13 as there is an increased sensitivity for C. acnes detection, however this is accompanied by a decrease in specificity.15 Indeed, we have detected that all strains growing after day 8 were considered contaminants. This temporal difference between contaminants and true pathogens has been described by almost all authors, and it needs to be considered also when the interpretation of cultures is performed, while no differences were detected in our study.3,14,15,19
We observed that most isolates grew between day 3 and 5, showing a Gaussian pattern. However, despite some contaminants appear after day 7, most appear before this day, so interpretation of these isolates needs to be performed carefully. Moreover, according with our results, if an isolate grow after day 8, interpretation of the cultures must be performed taking into account that they may be contaminants. The amount of CFU detected must be also taken into account when performing the interpretation of cultures, because contaminants show in most cases a scanty growth of a few colonies in the culture media.
In conclusion, our results support the idea that most clinically relevant isolates of Cutibacterium spp. are probably detected in the first 7 days of incubation, while most contaminants can also grow in this period, and a careful interpretation of the results must be performed. However, prolonged incubation must be necessary for detection of other organisms, and probably is mandatory for those patients with a clinical-analytical diagnosis of infection and negative cultures at the end of the first 7 days.