Interferon gamma release assay (IGRA) is used to detect latent tuberculosis prior to biological treatments in the context of suspected inflammatory rheumatism.
MethodsWe report the case of a 50-year-old woman with negative IGRA test before adalimumab introduction for presumed axial spondyloarthritis.
ResultsThe worsening of symptoms under treatment led to further investigations and the diagnostic of disseminated tuberculosis (TB) was later established with miliary and multiple bone locations such as spondylitis and sacroilitis. The patient's history revealed past exposure to tuberculosis. This observation illustrates the limitations of IGRA in such situation due to its variable performance for active TB diagnosis.
ConclusionMisdiagnosis is frequent in bone tuberculosis due to non-specific signs. We draw the attention to the importance of a global risk assessment prior to the introduction of biological treatment for suspected chronic inflammatory rheumatism and recall the risk factors for false-negative IGRA. An extended treatment course may be necessary after exposure to anti-TNF-alpha.
El ensayo de liberación de interferón gamma (IGRA) se utiliza para detectar tuberculosis latente antes de los tratamientos biológicos en el contexto de sospecha de reumatismo inflamatorio.
MétodosPresentamos el caso de una mujer de 50 años con IGRA negativo antes de la introducción de adalimumab por presunta espondiloartritis axial.
ResultadosEl empeoramiento de los síntomas bajo tratamiento llevó a nuevas investigaciones y posteriormente se estableció el diagnóstico de tuberculosis (TB) diseminada con localizaciones pulmonar y óseas múltiples como espondilitis y sacroilitis. La historia de la paciente reveló una exposición pasada a la TB. Esta observación ilustra las limitaciones del IGRA en tal situación debido a su rendimiento variable para el diagnóstico de la TB activa.
ConclusionesEl diagnóstico erróneo es frecuente en la TB ósea debido a signos inespecíficos. Llamamos la atención sobre la importancia de una evaluación de riesgo global antes de la introducción de un tratamiento biológico para la sospecha de reumatismo inflamatorio crónico, y recordamos los factores de riesgo para falsos negativos del IGRA. Puede ser necesario un curso de tratamiento prolongado después de la exposición al tratamiento anti-TNF-alfa.
Detection of latent tuberculosis infection (LTBI) by interferon gamma release assays (IGRA) such as QuantiFERON prior to initiation of biological treatment is an integral part of immune-mediated management of diseases such as spondyloarthritis (SpA).
We report a case of osteoarticular tuberculosis (TB) revealed after treatment with adalimumab despite negative IGRA screening.
Case reportA 50-year-old woman was admitted in our internal medicine department with fever, weight loss and asthenia.
Two months before, her rheumatologist diagnosed a seronegative axial ankylosing spondylitis (SpA) and prescribed an adalimumab treatment. Buttock and low back pain with morning stiffness suggested the diagnosis at that time. There were none of the extraspinal manifestations usually associated with SpA. She reported no relevant medical history. HLA-B27 test was negative. MRI showed undeniable unilateral sacroiliitis and lumbar CT scan revealed aspecific degenerative lesions. QuantiFERON®-TB Gold Plus (Qiagen) assay was negative.
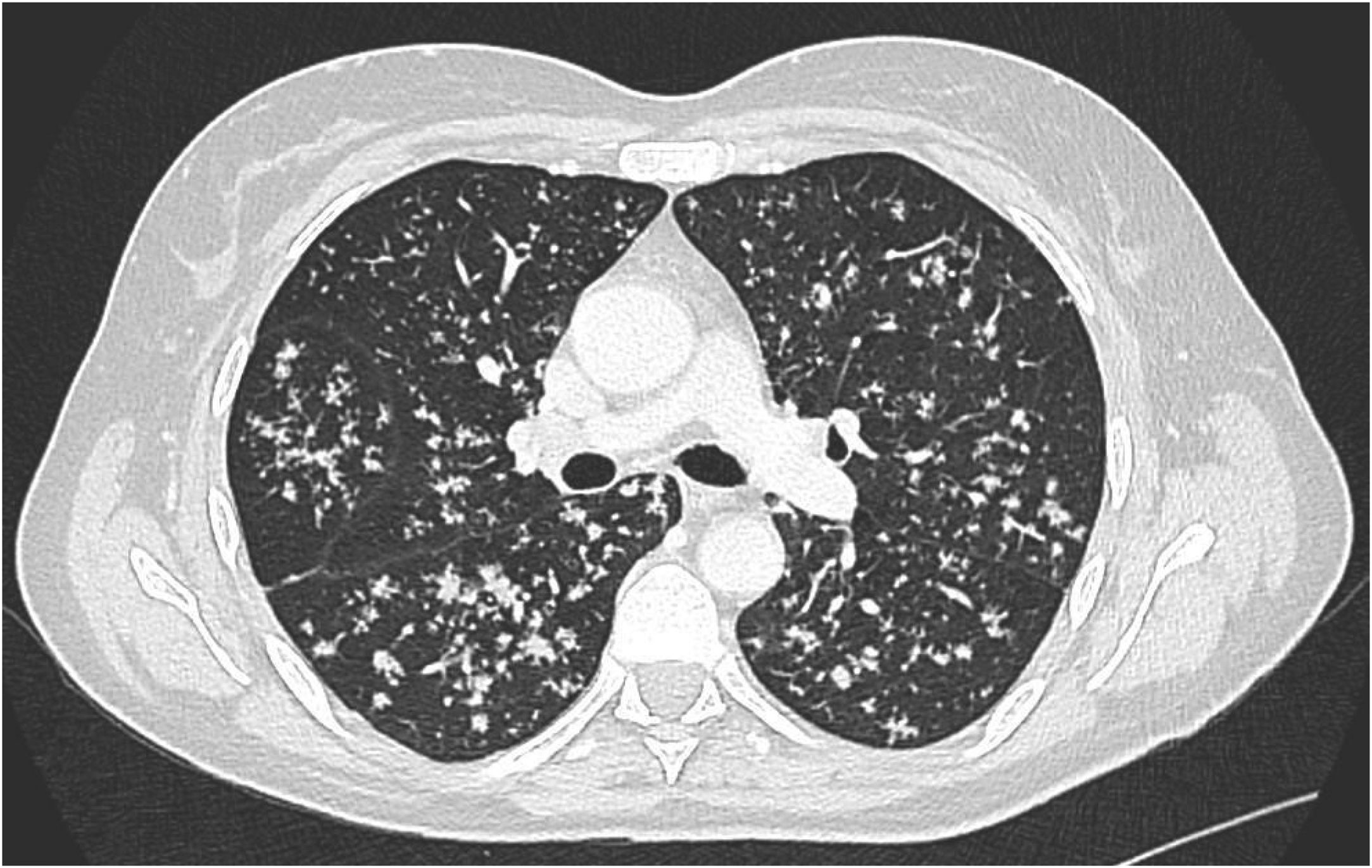
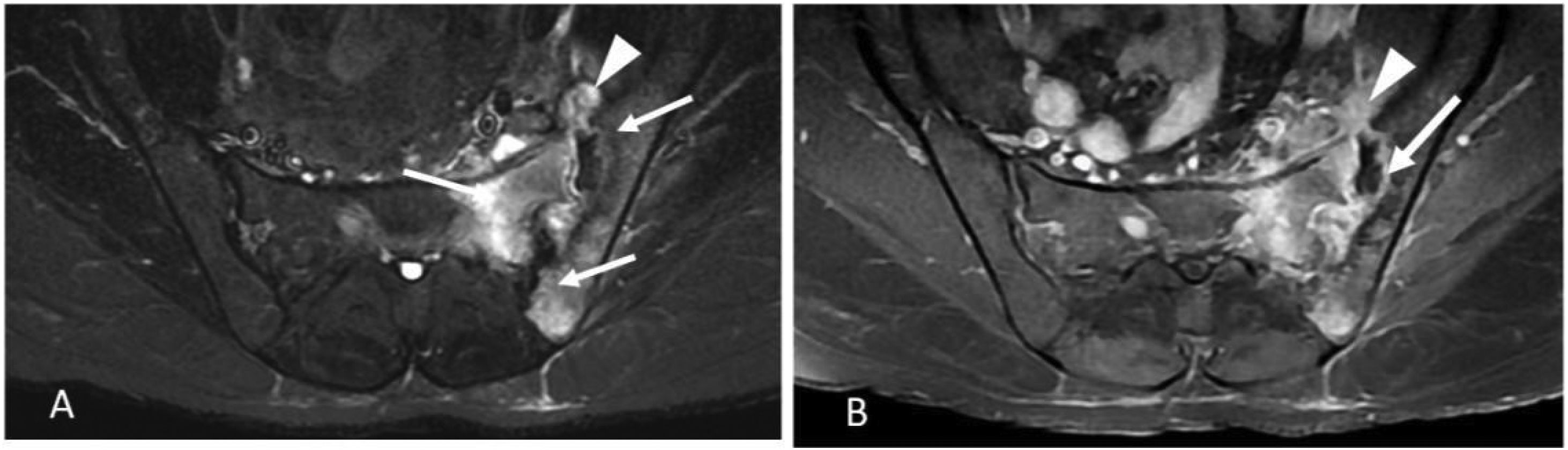
Response to non-steroidal anti-inflammatory drugs (NSAIDs) was poor and she was not treated with glucocorticoids. After two adalimumab injections (40mg every 14 days), she experienced fever, night sweats, 5kg weight loss, persistent cough and worse axial and hip pain than during the previous weeks. Her left sacroiliac joint was tender at palpation. CT scan revealed bilateral diffuse micronodular pneumopathy typical of miliary tuberculosis (TB) (Fig. 1), unilateral sacroilitis and an abscess within the piriformis muscle. MRI confirmed left sacroiliitis with contiguous abscess (Fig. 2), and spondylitis of L5 and to a lesser extent of T9, T8, T11 and T12 with adjacent anterior epidural space involvement. (18)F-FDG-PET/CT revealed strongly increased FDG uptake in both lungs, left iliac and pre-sacral nodes, left sacroiliac joint and several vertebral bodies. Laboratory findings showed only elevated CRP (45mg/l) with polyclonal hypergammaglobulinemia, while total lymphocytes count was 971/mm3.
MRI images showing left tuberculous sacroiliitis. Axial T2W fat-suppressed image (A) showing bone marrow edema (white arrows) and periarticular abscess (white arrowhead) involving the left sacroiliac joint. Axial T1W fat-suppressed contrast-enhanced images (B) showing extensive bone marrow edema, abscess in iliacus (white arrowhead), intraarticular abscess with joint space widening (white arrow).
X-ray-guided puncture was performed within the sacroiliac abscess. No acid-fast bacilli were observed on direct microscopic examination. Molecular detection of Mycobacterium tuberculosis complex by GeneXpert MTB/RIF® test was positive, without associated rifampicin resistance. Culture was positive for M. tuberculosis, exhibiting no antibiotic resistance. HIV serology was negative. A classical four drugs intensive phase (isoniazid, rifampin, pyrazinamide, and ethambutol) was initially started and then discontinued after 1 month due to a drug rash attributed to isoniazid and followed by a 4 drugs regimen combining levofloxacin, rifampin, pyrazinamide, and ethambutol. An adjuvant glucocorticoids treatment was given during 14 days at the time of the rash then discontinued. A prolonged treatment of 18 months was necessary to observe the compete regression of (18)F-FDG-PET/CT inflammatory lung and bone lesions despite persistent calcified lesions of the lung and destructive bone sequelae. No signs of relapse were observed after a 9 months follow-up period.
Those findings led us to interview the patient again; a significant family history was discovered, as the patient's father and two of her sisters had been diagnosed with tuberculosis in childhood.
To her knowledge, the patient did not receive any vaccination neither chemoprophylaxis or curative treatment for TB.
DiscussionIn the present case, axial spondyloarthritis was first suspected. Several points might have challenged this diagnosis: recent symptom onset in a patient over the age of 50, absence of classical extra-rheumatological SpA-associated features such as dactylitis, uveitis, enthesitis, psoriasis or Crohn's disease, inefficacy of NSAIDs, and negative HLA B27 status. We favor the hypothesis of a dramatic worsening of preexisting chronic osteoarticular tuberculosis after anti-TNF treatment.
The impact of anti-TNF alpha on the natural course of tuberculosis was soon reported during the first years of use. A 2017 metanalysis reported an OR of 1.94 (95% CI, 1.10–3.44; p=0.02) for developing TB under anti-TNF treatment.1 This risk was 26-fold greater than in the general population in a study carried out in a region where tuberculosis is highly endemic.2 An incidence of 9.62 TB cases per 1000 exposed patients was observed in patients receiving anti-TNF alpha in the recent review by Sartori et al.3 Given the persistent high risk of LTBI reactivation in this context, the World Health Organization recently reaffirmed the interest of screening for LTBI before initiating anti-TNF, advocating the use of IGRA.
Since the IGRA was negative and NSAIDs were ineffective, first-line anti-TNF treatment was proposed to our patient, with the initial assumption of SpA, in accordance with the French Society of Rheumatology guidelines for management of axial spondyloarthritis.4
IGRA performance depends on the context in which the test is used.
In a systematic review by Chang et al., with a 90% threshold among immunocompetent adults, QuantiFERON-TB positive likelihood ratio (PLR) was 48 and therefore considered the best test to diagnose LTBI, while both T-SPOT.TB and QuantiFERON-TB were able to exclude LTBI, with 0.10 and 1.11 negative likelihood ratios (NLR), respectively. Neither of these tests could be used to diagnose active TB and only T-SPOT.TB excluded the diagnosis, with a 0.18 NLR.5 In this study, estimated prevalence was 10–55% for LTBI in the context of contact investigation and 40–60% for TB among symptomatic patients, respectively. Mrozek et al. reported a 79% sensitivity for LTBI diagnosis using QuantiFERON versus 84% with T-SPOT.TB.5,6 In Diel's meta-analysis, the negative predictive value (NPV) of QuantiFERON and T-SPOT.TB for progression to tuberculosis among immunocompetent adults in low-incidence countries was 99.8% and 97.8%, respectively, while NPV was 94 and 88% for patients with active TB.7 In a systematic review including 3/22 studies from high-incidence countries, Pai et al. reported a pooled specificity for QuantiFERON among non-BCG vaccinated adults of 98% and 96% in vaccinated populations, versus 97% and 59% for tuberculin skin test.8
Individual data must be taken into account in case of negative IGRA, despite its high negative predictive value. In the case of TB disease, the impact on diagnostic strategy is much more delicate. IGRA was positive in 80% of TB disease in recent studies.9 Specificity was too low to distinguish between active and latent TB, and positivity was lower for miliary-type forms, up to 68–79%. However, it must be kept in mind that it should be used only for latent TB diagnosis rather than active TB.9,10
The false-negative rate of IGRA was 8–19% in the systematic review by Sester et al. in 2011, comparable to more recent series.7,9 Other authors reported high rates specifically for extra-pulmonary tuberculosis: 13.1% for Pan et al. and up to 28.8% for Kim et al., although the authors emphasized the superiority of IGRA over intradermoreaction (IDR) in this context.10,11 In a pediatric population of 270 patients, a combined approach using both tuberculin skin test and IGRA has been reported to identify more LTB cases than either of these tests alone.12 Therefore, this dual strategy might be suggested when the diagnostic suspicion remains high despite a negative single test.
Risk factors for false-negative IGRA in tuberculosis notably include advanced age (variously defined,11,13 and the only significant variable in the study by the Tuberculosis Network European Trials Group in 2015,14 low circulating lymphocyte count, HIV infection, use of T-SPOT.TB, BMI<16 or >25, active smoking, symptom duration>6 months, and bilateral pulmonary, bone or joint or meningeal involvement.11,13,15 Except from a decreased absolute lymphocytes count at admission which rose up to 1600/mm3 at the end of TB treatment course, our patient had none of these risk factors. Whether her age should be taken into account as a risk factor is uncertain since the cut-off value of advanced age definition varies widely among studies, ranging 40–65 years old.15
Among the existing avenues in this context, expansion of regulatory T lymphocytes of the CD4+CD25+FoxP3+phenotype, decrease in Vδ2 (+) T lymphocyte levels with increased expression of FasL in this subpopulation, and elevated IL-4 and IL-10 levels may account for an increased risk of anergy.16,17
Our patient's case was remarkable, with a rather rare clinical presentation of tuberculosis and a false-negative QuantiFERON test delayed the diagnosis and led to worsening of symptoms after contraindicated treatment.
Tuberculous sacroilitis (TBS) is rare and often associated with delayed diagnosis or misdiagnosis, as in the case of our patient. Skeletal TB accounts for 10% of extra-pulmonary TB and for 3–5% of all TB cases. While vertebral TB is the most frequent presentation, sacro-iliac joint involvement is <10%.18,19
Symptoms are non-specific. In a prospective study on 35 patients, low back pain and difficulty walking were the most frequent symptoms associated with TBS.19
Although mainly unilateral, the pain can be confusing and may affect the high, thigh or associated with sciatic nerve irritation. As reported herein, the disease course is slower than with pyogenic osteoarthritis and can be misdiagnosed with chronic inflammatory disease.
CT may reveal joint widening, margin sclerosis, bone destruction or sequestrum while MRI is useful to detect early TBS and to delimit soft tissue abscesses.18,19
Diagnosis confirmation relies on pathogen identification through direct examination and/or culture of needle aspiration material or tissue samples. Histological examination might be useful when culture is negative, revealing caseous necrosis within granulomatous lesions.
TBS is frequently associated with abscess in adjacent soft tissues that may spread in the hip joint.18
Following a prolonged antimicrobial treatment with a median duration of 12 months, infection healing is usual in skeletal TB but ankylosis is the expected long-term evolution of TBS.20 Accordingly, patients may report persisting discomfort but walking is usually not compromised. Although the treatment is often conservative, some patients may require surgical procedure for drainage, curettage of necrotic tissue and/or arthrodesis in severe cases, notably for joint instability or extensive and complicated abscesses.19
Our case suggests that an underlying chronic infection should be suspected when the evolution is poor during treatment with anti-TNF-alpha, notably TB, non-tuberculous mycobacteria and Tropherymawhipplei.21
In the context of suspected chronic inflammatory rheumatism, IGRA cannot replace overall assessment of LTBI risk. Risk factors for tuberculosis must be carefully assessed before starting anti-TNF treatment for chronic inflammatory rheumatism. One must keep in mind the variable performance of IGRA for active TB, depending on the impacted organ. Although anti-TNF treatment is currently recommended following negative IGRA results, it must be postponed if any suspicion of active TB remains.
Authors’ contributionsDD read and analyzed the papers used in the article and wrote the first draft of the manuscript.
CJL and MPO provided resources and reviewed the manuscript before submission.
CA contributed to the writing of the manuscript and proofread it before submission.
GD reviewed the manuscript before submission.
Data availabilityNot applicable.
Code availabilityNot applicable.
Ethics approvalAll procedures performed in this study were in accordance with the 1964 Helsinki declaration and its later amendments.
Consent to participateInformed consent was obtained from the patient included in the study.
Consent for publicationInformed consent was obtained from the patient to publish her data.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profits sector.
Conflict of interestsThe authors declare that they have no competing interests.