The anti-staphylococcal efficacy of cotrimoxazole in the setting of difficult-to-treat infections seems to be compromised by large amounts of pus and devitalized tissue, and, therefore, high levels of thymidine. Our objective was to evaluate the activity of cotrimoxazole against a staphylococcal foreign-body infection experimental model, which also yields significant quantities of thymidine.
Material and methodsWe used a rat tissue-cage model of infection (with high inherent thymidine levels) caused by a strain of methicillin-susceptible Staphylococcus aureus (MSSA; ATCC 29213). MIC values were determined (microdilution method) and compared in the presence or absence of tissue-cage fluid samples.
ResultsThe inefficacy of cotrimoxazole was found to be similar to that of the control group. The MIC of cotrimoxazole was 4–8 fold higher in the presence of rat tissue-cage fluid.
ConclusionsThe inefficacy of cotrimoxazole in our foreign-body infection model by MSSA, and the probable negative impact of the presence of thymidine on its efficacy, challenge the use of this drug in acute phases of foreign-body infections. It should be reserved as an alternative treatment when the infection is more controlled.
La eficacia antiestafilocócica del cotrimoxazol en el marco de las infecciones de difícil tratamiento parece alterarse por la presencia de grandes cantidades de pus y tejido desvitalizado que condicionan unos niveles elevados de timidina. Nuestro objetivo fue evaluar la actividad de cotrimoxazol en un modelo experimental de infección estafilocócica por cuerpo extraño, que también produce grandes cantidades de timidina.
Material y métodosUtilizamos un modelo de infección de caja subcutánea en rata (con elevados niveles inherentes de timidina) producida por una cepa de S. aureus sensible a meticilina (SASM; ATCC29213). Se determinaron los valores de CMI (método de microdilución) y se compararon en presencia del líquido de las cajas o sin él.
ResultadosCotrimoxazol mostró una ineficacia similar a la de un grupo control. El valor de su CMI aumentó de 4 a 8 veces en presencia del líquido de las cajas.
ConclusionesLa ineficacia de cotrimoxazol en nuestro modelo de infección de cuerpo extraño por SASM y el probable impacto negativo de la presencia de timidina en su eficacia, cuestionan el uso de este antibiótico en las fases agudas de estas. Por todo ello, cotrimoxazol debería reservarse como tratamiento alternativo cuando la infección esté ya más controlada.
High failure rates have been demonstrated in the treatment of serious Staphylococcus aureus infections with sulfonamides. Its combination with trimethoprim is synergistic and bactericidal. Consequently, trimethoprim–sulfamethoxazole (TMP–SMZ, cotrimoxazole) has been suggested as an alternative for the treatment of S. aureus infections. With regard to its anti-staphylococcal efficacy, previous studies have reported contradictory results, with variable failure rates in certain serious infections.1 Thus, comparisons of the efficacy of TMP–SMZ, vancomycin and beta-lactams for the treatment of staphylococcal bacteremia and endocarditis found TMP–SMZ to be the least effective.2 In the setting of osteoarticular infections, some studies have reported good results using TMP–SMZ alone or in combination with rifampicin, whereas other studies have noticed failure rates above 40% and emergence of resistant strains even when using TMP–SMZ at high doses for the treatment of prosthetic joint infections (PJI).3,4
A recent review of the use of TMP–SMZ against staphylococcal infections emphasized the importance of the presence of thymidine as a cause of the high failure rates observed in those infections in which large amounts of pus, damaged tissue and bacterial burden are prevalent.1 In the present study, we aimed to test the in vivo efficacy of TMP–SMZ against foreign-body infection by methicillin-susceptible S. aureus (MSSA), and to evaluate the extent to which it is affected by the presence of high amounts of thymidine. For these aims, we used a foreign-body infection model in rat, which provides high inherent serum thymidine levels (0.142–0.318μg/mL).5–7
Material and methodsMicroorganism and antibioticsA MSSA strain (ATCC29213) was used for all in vitro and in vivo studies. Trimethoprim and sulfamethoxazole were purchased from Sigma–Aldrich (Madrid) and Laboratorios Almofarma (Barcelona), respectively.
In vivo studiesThe experimental protocol complied with European (Directive 2010/63/EU) and Spanish (RD 53/2013) legislation on animal experimentation. The University of Barcelona's Ethics Committee for Animal Experiments approved the animal model previously standardized by our group.8 Briefly, the methodology consisted of subcutaneous implantation in rats of two Teflon tissue-cages with two polymethylmethacrylate coverslips (CV). After three weeks, the tissue cage fluid (TCF) was infected with MSSA. Three days later, TCF was obtained in order to quantify bacterial counts; therapy was then started and administered subcutaneously for seven days. After the end of treatment, TCF was recovered in order to count bacteria and animals were sacrificed. CVs were removed and processed as previously described.8 The criterion of efficacy was defined as a decrease in TCF bacterial counts between the beginning and end of treatment, and as the adherent bacteria counts on the CV. The appearance of resistant strains at the end of therapy was screened.
Pharmacokinetic studiesWe performed pharmacokinetic studies to select the equivalent human dose of 160mg TMP/800mg SMZ every 8h, which provide the ideal synergistic in vivo TMP:SMZ ratio of 1:20 found in human serum. Regarding the features of the tissue-cage infection model, we looked for the appropriate dosage of TMP–SMZ that could guarantee this 1:20 ratio in TCF.
Briefly, the dosage of 120mg/kg of TMP–SMZ (ratio 1:1, respectively) was subcutaneously administered to animals. Then, blood and TCF samples were collected at 0, 0.5, 1, 2, 4, 6, 8 and 24h after administration. Samples were centrifuged and serum was transferred into aliquots and stored at −20°C up to the time of analysis. For the precipitation of serum or TCF protein, trichloroacetic acid solution (50%, 20μL) was added to 200μL of serum or TCF samples (calibration and rats) and centrifuged at 18,000×g for 10min. The supernatants were collected and 5μL was injected to measure the drug concentration.
The concentrations of TMP and SMZ were analyzed using UHPLC.9 Chromatography was performed using a Waters Acquity®TM UHPLC system (Waters, MA, USA) with an ultraviolet detector. The separation was carried out with Acquity C18BEH™ (2.1×100mm id, 1.7μm, Waters, MA, USA). Elution was performed with a mobile phase solution of di-potassium hydrogen phosphate water solution (pH 7.2; 10mM) containing acetonitrile (20:80). The retention times of TMP and SMZ were 1.4 and 2.7min respectively. TMP was detected at 270nm, and SMZ at 254nm.
In vitro studies: assessment in the presence of thymidineFollowing the standard procedures, we determined the MIC value of TMP–SMZ (ratio 1:20) using Mueller Hinton Broth (MHB) and microdilution method.
To assess the effect of thymidine on TMP–SMZ activity, we used TCF samples recovered from pharmacokinetic studies (see above), which contained known concentrations of TMP–SMZ and inherent thymidine from rat. The specific thymidine concentration in the TCF samples was not determined, so its possible negative effect on cotrimoxazol efficacy was indirect evaluated by assuming the inherent high concentrations of thymidine in rats.5 Following the microdilution methodology, we determined the MIC value using a mixture of MHB and this TCF sample (ratio 1:1).
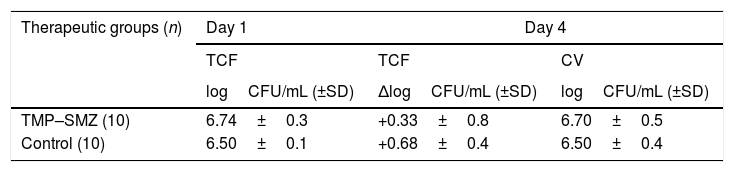
ResultsThe treatment with TMP–SMZ had to be suspended on the fourth day due to animals presented intolerance to the antibiotic. This therapy did not show significant differences with respect to the control group in terms of efficacy (Table 1). No resistant strains to TMP–SMZ were detected. During treatment with TMP–SMZ the animals showed polyuria and less reactivity. Our hypothesis that this was due to renal impairment associated with high levels of SMZ was not confirmed, since serum creatinine levels in treated and control rats did not differ (0.48mg/L and 0.42mg/L, respectively).
Bacterial counts from tissue-cage fluid (TCF) at day 1, and results of the efficacy of therapeutics groups against bacteria from TCF (decreases of bacterial counts at day 4) and from cover-slips (CV; bacterial counts at day 4).
| Therapeutic groups (n) | Day 1 | Day 4 | |
|---|---|---|---|
| TCF | TCF | CV | |
| logCFU/mL (±SD) | ΔlogCFU/mL (±SD) | logCFU/mL (±SD) | |
| TMP–SMZ (10) | 6.74±0.3 | +0.33±0.8 | 6.70±0.5 |
| Control (10) | 6.50±0.1 | +0.68±0.4 | 6.50±0.4 |
There were not significant differences between control and TMP–SMZ group.
Abbreviations: TMP-SMZ, cotrimoxazole; n, number of tissue cages; CFU, colony forming unit; ΔlogCFU/mL, decrease in logCFU/mL with respect to day 1.
Based on pharmacokinetic studies, we finally selected a dose of 120mg/kg/day of TMP–SMZ (proportion 1:1) that provide the ideal ratio TMP–SMZ of 1:20 in TCF. Concentrations of TMP and SMZ in TCF were similar to those achieved in human: Cmax (mg/L) and AUC0–24h (mgh/L): 3.74 and 45.83; 84 and 1569 respectively.
We observed that the in vitro MIC of TMP–SMZ in the absence of thymidine was 0.064mg/L, but in the presence of TCF the MICs were 4–8 fold higher.
DiscussionWe showed the ineffectiveness of TMP–SMZ in our animal model, a finding that was in agreement with previous reports conducted in murine models. While particular thymidine levels in TCF samples were not determined, we assumed that the presence of inherent high amounts of thymidine in murine serum may interfere with the in vivo activity of TMP–SMZ and may explain partially its ineffectiveness.
We wondered how relevant these observations might be for humans, in whom thymidine levels are low or absent in serum but may be higher in particular situations.5 Proctor et al. stressed the high TMP–SMZ failure rates observed in those MRSA infections in which large amounts of pus, damage tissue and bacterial burden are frequent, presumably due to the presence of thymidine.1 In a recent study by Zander et al., a median of 530μg/L of thymidine was detected in sputa of ten cystic fibrosis patients. These authors demonstrated that the activity of cotrimoxazole, as well as its synergy with rifampicin, was inhibited with 200mg/L of thymidine.10 Moreover, Stokes et al. reported that the required concentration of thymidine to inhibit the in vitro activity of cotrimoxazole was as low as 0.05mg/L.11
In this regard, the efficacy of cotrimoxazole seems to depend on the balance between the drug and thymidine levels found at the infection site. Additionally, its particular pharmacokinetic could be detrimental to TMP–SMZ efficacy in specific settings because the synergistic activity is based on attaining a ratio of 1:20, respectively. While this proportion is usually found in serum and interstitial human fluids, the final scenario may be different in osteoarticular and biofilm-related infections, where this ratio may not be warranted in bone or the intracellular milieu.12
In the setting of staphylococcal device-related osteoarticular infections, the pharmacokinetic interactions between cotrimoxazole and rifampicin are of particular concern and they may affect cotrimoxazole's final efficacy. Rifampicin is usually required due to its main role in these infections but it leads to decrease the levels of TMP–SMZ,9 and the clinical consequences of these interactions in the management of PJI have not been well evaluated.
The translation of results from experimental studies to clinical practice should be always done with caution. In fact, our experimental results, as well as the inconveniences of TMP–SMZ related to its pharmacokinetic particularities, are partially in contrast with the global good results reported using cotrimoxazole in clinical practice against staphylococcal device-related osteoarticular infections. In this way, our group showed a high efficacy of rifampin-cotrimoxazole combination in the setting of chronic osteomyelitis,13 and Stein et al. observed also a reasonable efficacy using high doses of cotrimoxazole in monotherapy against different osteoarticular infections.3 However, in both studies the risk of therapeutic failures was related to the foreign-body maintenance3,13 and, in some cases treated with monotherapy, cotrimoxazole-resistant strains appeared.3 Overall, these findings could indicate the limitations of TMP–SMZ efficacy in some difficult-to-treat infections, but in contrast, a better outcome may be achieved whether patients receive this therapy for long time and after the high bacterial burden at the early phase of infection has been diminished.
The main limitation of this work was that specific thymidine levels in TCF samples were not determined. However, the negative impact of thymidine on cotrimoxazole efficacy may be reasonably assumed on the basis of the inherent existence of high amount of thymidine in rats,5 and the fact that we observed indirect evidence of the decrease in the cotrimoxazole activity in TCF samples. In addition, the inability to increase the TMP–SMZ dosage or to use longer treatments due to the rats’ intolerance meant that we were unable to carry out further studies with these animals, which might have increased the final efficacy. Nevertheless, we achieved acceptable TMP–SMZ levels in TCF and, based on previous experiences using the tissue-cage model infection, which have usually evaluated the antimicrobial efficacy of therapies over 3–7 days, we would not expect a significant improvement in TMP–SMZ efficacy.8
In conclusion, TMP–SMZ treatment was ineffective against foreign-body infection by MSSA. Our findings are in agreement with previous reports of clinical failure with TMP–SMZ in device-related infections, and they illustrate to some extent the negative impact of the presence of thymidine on the efficacy of cotrimoxazole. Caution should be taken when TMP–SMZ is used in suppurating infections, due to its apparent inhibition by thymidine. In the specific case of staphylococcal PJI managed with implant retention, the rifampicin-cotrimoxazole combination should be avoided during the acute phase of the infection and should be reserved as an alternative treatment at a later point when the infection is more controlled.
FundingThis study was supported by a research grant from the Ministerio de Sanidad y Consumo, ISCIII (PI13/00550), and by Plan Nacional de I+D+i and ISCIII, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD12/0015) – co-financed by European Development Regional Fund “A way toachieve Europe” ERDF.
Conflicts of interestThe authors have no conflicts of interest.
We thank to P. Fontova (Nephrology Service and Laboratory of Experimental Nephrology, University of Barcelona, Campus Bellvitge) for his assistance in the UHPLC method used.