With the widespread introduction of conjugate meningococcal and pneumococcal vaccines, the prevalence and etiology of invasive bacterial infections have changed. We aimed to review all cases of bacteremia in a level II pediatric department over a ten-year period in the post-pneumococcal conjugate vaccine era.
MethodsWe reviewed all positive blood cultures (BC) obtained in our department between 2007 and 2016. Results were classified as contaminants, potential pathogens or confirmed pathogens, based on species, number of positive BC in the episode and the patients’ medical history. Demographic and clinical data were collected for patients with identified pathogens.
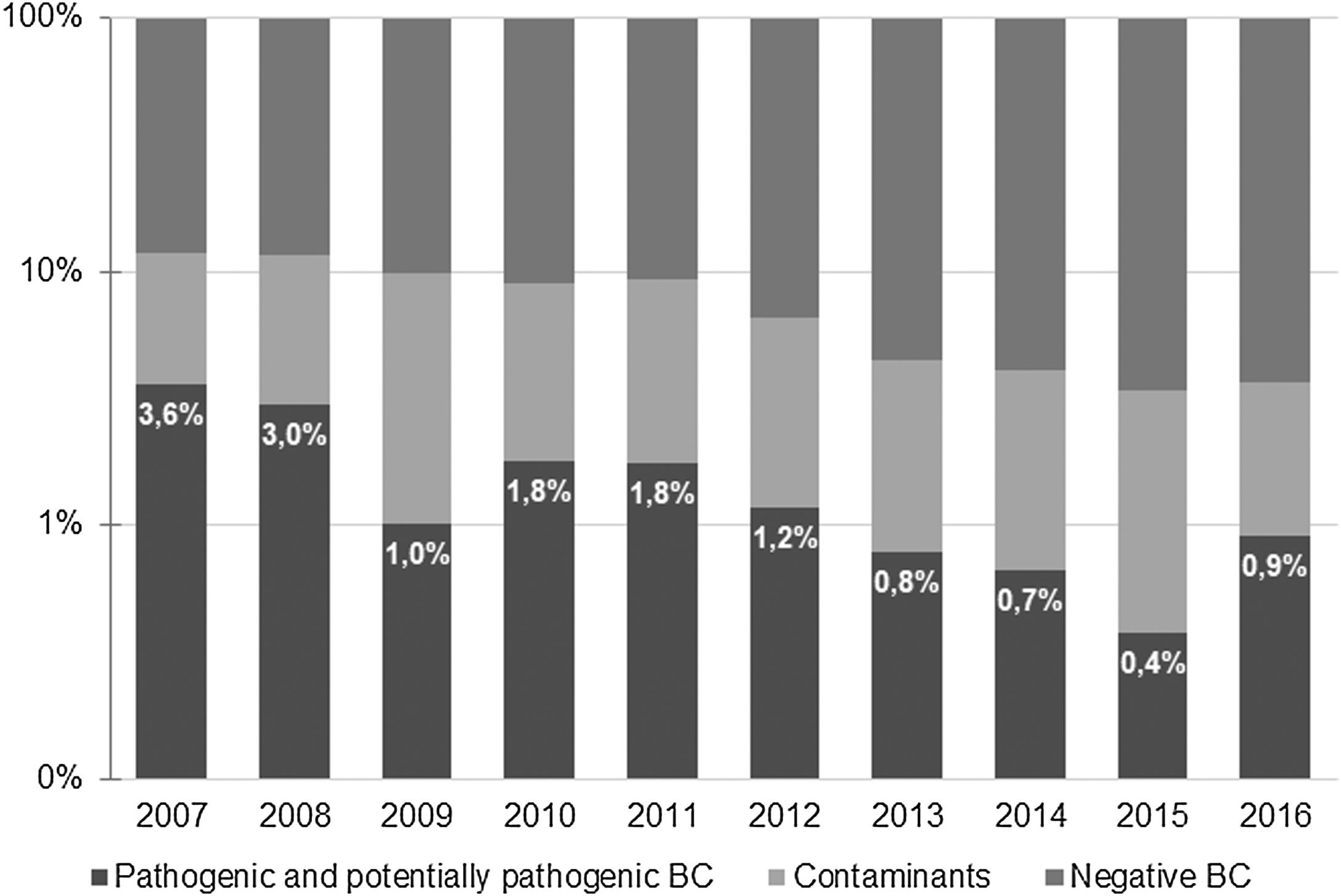
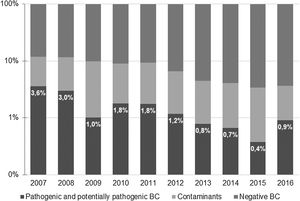
ResultsA total of 638 positive BC were identified (6.6% of total BC); 120 (1.2%) were considered to represent true bacteremia. The most frequently identified microorganism was Streptococcus pneumoniae (29.2%), with a decrease in the number of cases between 2008 and 2015. Staphylococcus aureus was the second most common organism (19.2%) being 21.7% of these methicillin-resistant. Escherichia coli was the most common isolate in children aged less than three months.
ConclusionWe found a rate of true bacteremia in children similar to recent studies. Although Streptococcus pneumoniae remains the most common microorganism, its prevalence may be declining. Monitoring microbiological data in children has implications in practice, particularly in local antibiotic prescription.
Con la introducción generalizada de las vacunas conjugadas meningocócicas y neumocócicas, la prevalencia y la etiología de las infecciones bacterianas invasivas han cambiado. Nuestro objetivo fue revisar todos los casos de bacteriemia en un departamento de pediatría de nivel II durante un período de 10 años en la era de la vacuna conjugada posneumocócica.
MétodosRevisamos todos los hemocultivos (HC) positivos obtenidos en nuestro departamento entre 2007 y 2016. Los resultados se clasificaron como contaminantes, patógenos potenciales o patógenos confirmados, según la especie, el número de HC positivos en el episodio y la historia clínica de los pacientes. Se recopilaron datos demográficos y clínicos de pacientes con patógenos identificados.
ResultadosSe identificaron un total de 638 HC positivos (6,6% del total de HC); se consideró que 120 (1,2%) representaban una bacteriemia verdadera. El microorganismo identificado con mayor frecuencia fue Streptococcus pneumoniae (29,2%), con una disminución en el número de casos entre 2008 y 2015. Staphylococcus aureus fue el segundo aislado más común (19,2%); El 21,7% eran resistentes a la meticilina. Escherichia coli fue el aislado más común en niños menores de 3 meses.
ConclusiónEncontramos una tasa de bacteriemia verdadera similar a la de estudios recientes. Aunque el Streptococcus pneumoniae sigue siendo el microorganismo más común, su prevalencia puede estar disminuyendo. El seguimiento de datos microbiológicos como este tiene implicaciones en la práctica, especialmente en la prescripción local de antibióticos.
Fever is one of the most common presentations requiring medical observation in pediatric patients. Although in most cases it is caused by a benign and self-limited viral infection, sometimes it is the sole presenting sign of an invasive bacterial infection.1–3
Blood cultures (BC) remain the gold standard for diagnosing bacteremia.4 However, there is no universal consensus regarding indications for BC collection in the febrile child.5 Most commonly they are obtained during the investigation of an ill-appearing child or in immunocompromised patients, but also in well-appearing children presenting with fever without source (FWS) considered to be at high risk of occult bacteremia (OB).6,7 BC are also frequently performed as part of the complementary evaluation of certain focal infections, particularly in patients that require parenteral antibiotic therapy.5–7
Over the last three decades, the development of vaccines against Haemophilus influenzae, Streptococcus pneumoniae and Neisseria meningitidis has led to changes in the epidemiology of invasive bacterial infections.5,8,9 In our country, the Haemophilus influenzae type b (Hib) vaccine was introduced in the national vaccination program (universal and free of charge) in 2000. The meningococcal type C (MenC) vaccine became commercially available in 2001 and was later introduced in our national vaccination program in 2006. The first vaccine against Streptococcus pneumoniae to become commercially available was the heptavalent pneumococcal conjugate vaccine (PCV-7) in 2001, followed by the 10-valent pneumococcal conjugate vaccine (PCV-10) in 2009 and the 13-valent pneumococcal conjugate vaccine (PCV-13) in 2010. The PCV-13 was then introduced in our national vaccination program in 2015. The meningococcal serogroup B (MenB) vaccine became commercially available in 2014 and was introduced in our national vaccination program in 2020. The meningococcal conjugate vaccine against serogroups A, C, W and Y (MenACWY) is commercially available since 2010 and is currently recommended on an individual basis. In this new setting, it is increasingly challenging to decide which well-appearing, previously healthy children with FWS require diagnostic evaluation.5
In this study, we aimed to: (1) identify all positive BC obtained in our department over a ten-year period in the post-pneumococcal conjugate vaccine era; (2) describe identified pathogenic agents and their antimicrobial susceptibility; (3) describe the diagnoses associated with bacteremia.
MethodsOur study was conducted in a level II hospital located in Viseu, Portugal. The local pediatric department is comprised of a Pediatric Ward (mean 1255 admissions per year, including surgical but not oncological patients), a Neonatology Unit (without intensive care) and a Pediatric Emergency Department (mean 34,000 admissions per year).
We performed a retrospective analysis of all positive BC obtained in the Pediatric Emergency Department and Pediatric Ward in children aged 29 days to 17 years and 364 days, over a ten-year period between 2007 and 2016.
For that purpose, a list of all positive BC collected in this population was obtained from the local Department of Clinical Pathology, including isolated microorganisms and their antimicrobial susceptibility. Due to the unavailability of stored data from the first five months of 2007, our study included BC collected between June 1st 2007 and December 31st 2016. The total number of BC collected in this population was also obtained from the same records. Only one positive BC was considered per disease episode if there was more than one BC identifying the same agent.
At our institution, BC are processed manually and reviewed daily; if no growth occurs after five days, they are considered negative and discarded. Becton Dickinson BBL™ prepared media are used for culture, and identification is performed using an automated method (Vitek®). Susceptibility testing and reporting was performed according to the Clinical & Laboratory Standards Institute (CLSI) guidelines until 2010, and since then following the European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendations.
Positive BC were then classified into three groups: (1) pathogenic; (2) potentially pathogenic; and (3) contaminants. BC identifying microorganisms known to consistently cause human disease were classified as pathogenic.10,11 If microorganisms classically considered to be contaminants were identified more than once in the same episode or in patients with a known immunodeficiency or indwelling medical devices, BC were classified as potentially pathogenic. These criteria have been previously used in similar studies.6,7,11–15 Otherwise they were classified as contaminants.
Patients with positive BC for pathogenic or potentially pathogenic microorganisms were considered to have true bacteremia. Medical records from these patients were then analyzed and data was collected regarding patient's gender, age, immunization status, diagnosis, antimicrobial therapy and outcome. The main diagnosis on discharge was considered for study purposes. Primary bacteremia with sepsis was recorded when BC identified a pathogen or potential pathogen in an ill-appearing child presenting with sepsis and no identifiable focal infection. OB was recorded when BC identified a pathogen or potential pathogen in a well-appearing febrile child with no identifiable focal infection.
Statistical analysis was performed using IBM SPSS Statistics 24®. Categorical data are presented as frequencies and percentages. Continuous data are presented as median and interquartile range.
Approval from our institutions’ ethics committee was granted for the study.
ResultsA total of 9621 BC were obtained during the study period. Of those, 638 (6.6%) were positive −518 (5.4%) identified microorganisms classified as contaminants and 120 (1.2%) were considered to represent true bacteremia. Most positive BC were obtained at the Pediatric Emergency Department (379, 59.4%), and the remaining 259 (40.6%) were collected at the Pediatric Ward. The percentage of true bacteremia decreased from 3.6% in 2007 to 0.9% in 2016 and remained below 1% since 2013 (Fig. 1).
The 120 episodes of bacteremia occurred in 118 patients; 75 (63.6%) were male. Median age at diagnosis was 22.5 months (interquartile range 7–78.5 months), with 55.0% of patients aged less than 36 months and 10.3% aged less than 3 months. Eight patients had risk factors for bacteremia – four were immunocompromised (one patient with C7 deficiency; one under investigation for possible T cell deficiency, one with recurrent neutropenia of unknown cause, and one with chronic pancytopenia due to portal cavernoma with portal hypertension) and four had an indwelling medical device (one central venous catheter, one pacemaker and two ventriculoperitoneal shunts).
Microorganisms causing true bacteremiaThe most frequently identified microorganisms were Streptococcus pneumoniae (29.2%), Staphylococcus aureus (19.2%) and Escherichia coli (11.7%). Streptococcus pneumoniae was the most common pathogen isolated in children aged three months or older, while in those aged less than three months the most common pathogen was Escherichia coli (Table 1). Seven blood cultures (5.8%) grew potentially pathogenic agents, all of them coagulase negative staphylococci (CoNS) – three cases occurred in immunocompromised patients, two in patients with indwelling medical devices and two in previously healthy children with two positive BC for the same agent during the same episode.
Microorganisms causing bacteremia (total and by age group).
| Total(n=120) | <3 months(n=13) | 3–36 months(n=53) | ≥36 months(n=54) | |
|---|---|---|---|---|
| Streptococcus pneumoniaed | 35 (29.2%) | 2 (15.4%) | 15 (28.3%) | 18 (33.3%) |
| Staphylococcus aureus | 23 (19.2%) | 1 (7.7%) | 6 (11.3%) | 16 (29.6%) |
| Escherichia coli | 14 (11.7%) | 3 (23.1%) | 9 (17.0%) | 2 (3.7%) |
| Neisseriameningitidisa | 10 (8.3%) | 2 (15.4%) | 4 (7.5%) | 4 (7.4%) |
| Haemophilus influenzaed | 6 (5.0%) | – | 4 (7.5%) | 2 (3.7%) |
| Salmonella spp. | 4 (3.3%) | – | 3 (5.7%) | 1 (1.9%) |
| Streptococcus pyogenes | 4 (3.3%) | – | 1 (1.9%) | 3 (5.6%) |
| Streptococcus agalactiae | 3 (2.5%) | 1 (7.7%) | 2 (3.8%) | – |
| Campylobacter jejuni | 2 (1.7%) | – | – | 2 (3.7%) |
| Enterococcus faecalis | 2 (1.7%) | 2 (15.4%) | – | – |
| Klebsiella pneumoniae | 2 (1.7%) | – | 2 (3.8%) | – |
| Enterobacter cloacae | 1 (0.8%) | – | – | 1 (1.9%) |
| Enterobacter asburiae | 1 (0.8%) | – | 1 (1.9%) | – |
| Kingella kingae | 1 (0.8%) | – | 1 (1.9%) | – |
| Nocardia spp. | 1 (0.8%) | – | 1 (1.9%) | – |
| Pasteurella multocida | 1 (0.8%) | – | 1 (1.9%) | – |
| Proteus mirabilis | 1 (0.8%) | – | – | 1 (1.9%) |
| Pseudomonas aeruginosa | 1 (0.8%) | – | 1 (1.9%) | – |
| Candida parapsilosisb | 1 (0.8%) | – | – | 1 (1.9%) |
| Coagulase negative staphylococci (CoNS) c | 7 (5.8%) | 2 (15.4%) | 2 (3.8%) | 3 (5.6%) |
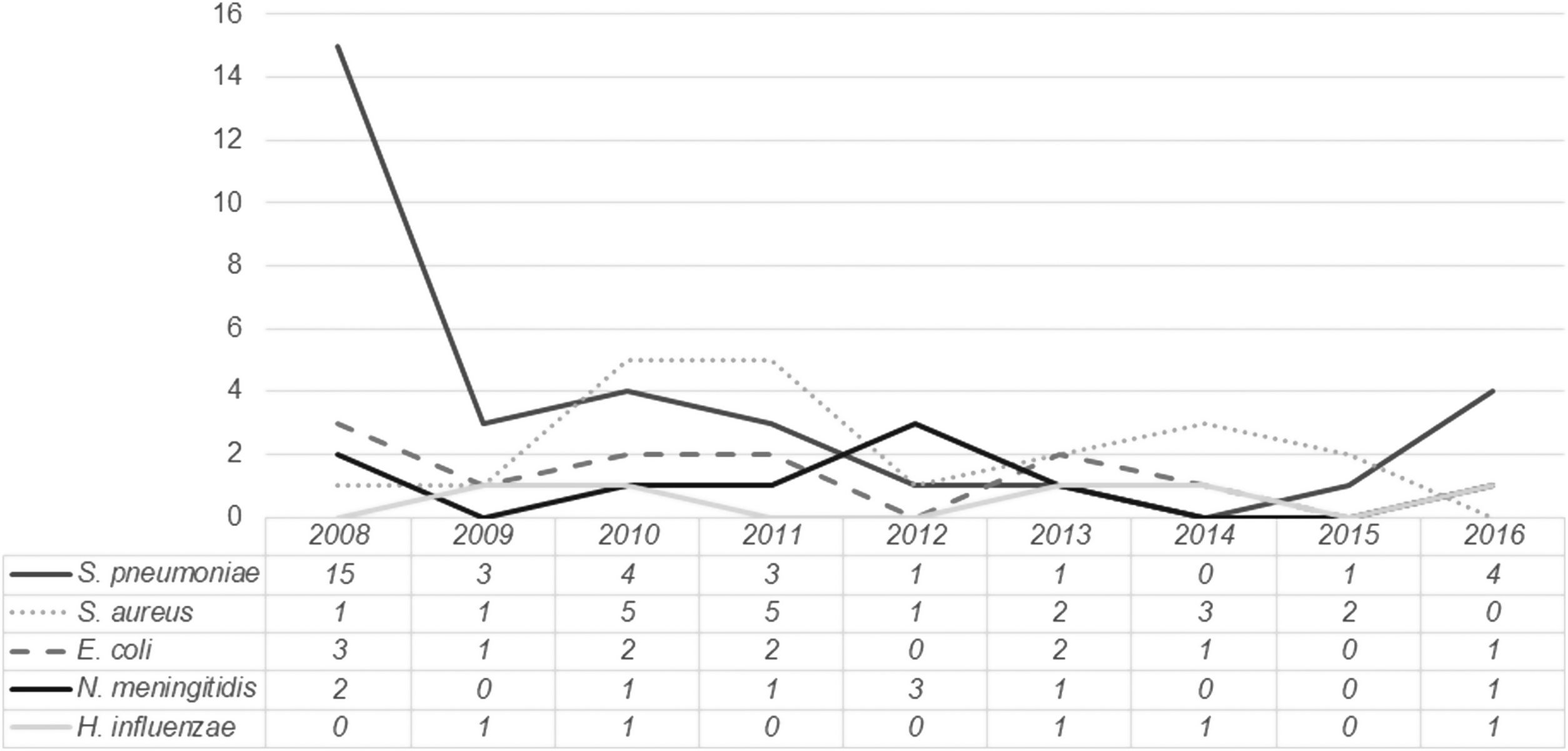
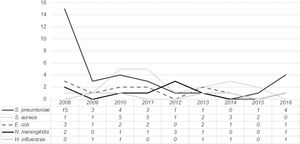
The distribution of the most frequently isolated pathogens during the study period is presented in Fig. 2. While for most pathogens no clear trend is identified, for Streptococcus pneumoniae a sustained decline occurred between 2008 and 2015, followed by a slight increase in 2016.
Antimicrobial susceptibility data was analyzed for the most commonly isolated pathogens. All Streptococcus pneumoniae isolates were susceptible to penicillin. Five Staphylococcus aureus isolates were methicillin-resistant (21.7%). One case met criteria for healthcare-associated methicillin-resistant Staphylococcus aureus (HA-MRSA) (hospitalization for more than 48h before culture), while the remaining four were community-associated (CA-MRSA). Escherichia coli isolates were resistant to ampicillin in 42.9% of cases, to the association of amoxicillin and clavulanic acid in 21.4% of cases, and to second generation cephalosporins in one case (7.1%). All Neisseria meningitidis and Haemophilus influenzae isolates were susceptible to third generation cephalosporins.
Immunization statusIn children with bacteremia due to Streptococcus pneumoniae, 14 (40.0%) had not received any dose of any pneumococcal conjugate vaccine (information could not be retrieved for two patients); 9 (27.3%) had received at least two doses of PCV-7 and 9 (27.3%) had received at least two doses of PCV-13. Information regarding Streptococcus pneumoniae serotyping was not available as it is not routinely performed at the local laboratory.
No serogroup C Neisseria meningitidis isolates were identified, although information was not available for four cases. Of those, three (75.0%) had received at least one dose of the MenC vaccine and none had received any dose of the MenB vaccine. Of those with bacteremia due to serogroup B Neisseria meningitidis, none had received any dose of the MenB vaccine.
All children with bacteremia due to Haemophilus influenzae had received at least two doses of the Hib vaccine. Information regarding Haemophilus influenzae serotyping was also unavailable as it is not routinely performed at the local laboratory.
Diagnosis associated with bacteremiaThe most common diagnosis associated with bacteremia were pneumonia (28.3%), OB (16.7%) and osteoarticular infections (12.5%). However, in infants aged less than three months, the most common diagnosis was UTI (30.8%) (Table 2). Table 3 displays the most frequently isolated pathogens for the most common diagnoses.
Diagnosis associated with bacteremia (total and by age group).
| Total(n=120) | <3 months(n=13) | 3–36 months(n=53) | ≥36 months(n=54) | |
|---|---|---|---|---|
| Pneumonia | 34 (28.3%) | 3 (23.1%) | 12 (22.6%) | 19 (35.2%) |
| Occult bacteremia | 20 (16.7%) | 2 (15.4%) | 11 (20.8%) | 7 (13.0%) |
| Osteoarticular infections | 15 (12.5%) | – | 2 (3.8%) | 13 (24.1%) |
| Urinary tract infection (UTI) | 13 (10.8%) | 4 (30.8%) | 8 (15.1%) | 1 (1.9%) |
| Meningitis | 12 (10.0%) | 1 (7.7%) | 7 (13.2%) | 4 (7.4%) |
| Primary bacteremia with sepsis | 8 (6.7%) | 2 (15.4%) | 4 (7.5%) | 2 (3.7%) |
| Endocarditis | 3 (2.5%) | – | 2 (3.8%) | 1 (1.9%) |
| Acute gastroenteritis | 3 (2.5%) | – | 2 (3.8%) | 1 (1.9%) |
| Acute otitis media | 2 (1.7%) | – | 2 (3.8%) | – |
| Cellulitis | 2 (1.7%) | – | 2 (3.8%) | – |
| Acute appendicitis | 1 (0.8%) | – | – | 1 (1.9%) |
| Acute sinusitis | 1 (0.8%) | – | – | 1 (1.9%) |
| Bronchiolitis | 1 (0.8%) | 1 (7.7%) | – | – |
| Central venous catheter-associated bloodstream infection | 1 (0.8%) | – | – | 1 (1.9%) |
| Endarteritis | 1 (0.8%) | – | – | 1 (1.9%) |
| Intra-abdominal abscess | 1 (0.8%) | – | – | 1 (1.9%) |
| Perianal abscess | 1 (0.8%) | – | 1 (1.9%) | – |
| Varicella | 1 (0.8%) | – | – | 1 (1.9%) |
Microorganisms causing bacteremia for the most commonly identified diagnosis.
| Microorganisms causing bacteremia (n; %) | |
|---|---|
| Pneumonia(n=34) | Streptococcus pneumoniae (25; 73.5%)Staphylococcus aureus (4; 11.8%)Haemophilus influenzae (3; 8.8%)Salmonella spp. (1; 2.8%)CoNSa (1; 2.8%) |
| Occult bacteremia(n=20) | Streptococcus pneumoniae (3; 15.0%)Staphylococcus aureus (3; 15.0%)Escherichia coli (2; 10.0%)Enterobacter cloacae (1; 5.0%)Enterobacter asburiae (1; 5.0%)Kingella kingae (1; 5.0%)Klebsiella pneumoniae (1; 5.0%)Pasteurella multocida (1; 5.0%)Salmonella spp. (1; 5.0%)Streptococcus agalactiae (1; 5.0%)CoNSa (5; 25.0%) |
| Osteoarticular infections(n=15) | Staphylococcus aureus (12; 80.0%)Streptococcus pyogenes (3; 20.0%) |
| Urinary tract infection (UTI)(n=13) | Escherichia coli (11; 84.6%)Proteus mirabilis (1; 7.7%)Streptococcusagalactiaeb (1; 7.7%) |
| Meningitis(n=12) | Neisseria meningitidis (7; 58.3%)Streptococcus pneumoniae (4; 33.3%)Haemophilus influenzae (1; 8.3%) |
| Primary bacteremia with sepsis(n=8) | Neisseria meningitidis (3; 37.5%)Campylobacter jejuni (1; 12.5%)Enterococcus faecalis (1; 12.5%)Klebsiella pneumoniae (1; 12.5%)Streptococcus agalactiae (1; 12.5%)CoNSa (1; 12.5%) |
a Potentially pathogenic agents.
Medical records could not be retrieved for five episodes, and therefore information regarding clinical management was either missing or incomplete, and they were excluded from further analysis. Eighteen patients (15.7%) received no antimicrobial therapy. This included one previously healthy 1-month old infant with bacteremia due to Enterococcus faecalis with no identifiable focal infection who was admitted in the Emergency Department in cardiorespiratory arrest and died before starting antimicrobial therapy. All other patients had a favorable outcome (Table 4). Of those who received antimicrobial therapy, 83 (85.6%) were started empirically after BC collection, 11 (11.3%) were started only after the BC became positive and 3 (3.1%) were already receiving antimicrobial therapy at the time of BC collection. All had favorable outcomes.
Characteristics and outcomes of the patients who did not receive antimicrobial therapy.
| Gender | Age | Identified pathogen | Clinical diagnosis | Reason for not receiving antimicrobial therapy | Outcome | |
|---|---|---|---|---|---|---|
| 1 | M | 4 y | Campylobacter jejuni | Acute gastroenteritis | b | Favorable |
| 2 | M | 2 mo | CoNS | Occult bacteremiaa | b | Favorable |
| 3 | M | 2 mo | CoNS | Occult bacteremiaa | b | Favorable |
| 4 | M | 9 mo | CoNS | Occult bacteremiaa | b | Favorable |
| 5 | M | 5 y | CoNS | Occult bacteremiaa | b | Favorable |
| 6 | M | 1 mo | E. faecalis | Sepsis | Admitted on cardiorrespiratory arrest, died before starting antibiotics | Death |
| 7 | M | 2 mo | E. faecalis | Acute bronchiolitis | b | Favorable |
| 8 | M | 22 mo | Kingella kingae | Occult bacteremiaa | b | Favorable |
| 9 | M | 11 mo | Pasteurella multocida | Occult bacteremiaa | b | Favorable |
| 10 | M | 23 mo | Salmonella spp. | Viral pneumonia | b | Favorable |
| 11 | M | 21 mo | Salmonella spp. | Acute gastroenteritis | b | Favorable |
| 12 | F | 7 mo | S. aureus | Occult bacteremiaa | b | Favorable |
| 13 | F | 8 y | S. aureus | Uncomplicated varicella | b | Favorable |
| 14 | M | 8 mo | S. aureus | Occult bacteremiaa | b | Favorable |
| 15 | F | 13 y | S. aureus | Occult bacteremiaa | b | Favorable |
| 16 | M | 7 y | S. aureus | Septic arthritis | Reevaluation after completing a full course of flucloxacillin for septic arthritis. Afebrile and clinically well. | Favorable |
| 17 | M | 5 mo | S. pneumoniae | Occult bacteremiaa | a | Favorable |
| 18 | M | 7 y | S. pneumoniae | Acute appendicitis | Favorable |
Legend: F – female; M – male.
We aimed to review all positive BC obtained in our department during an approximately ten-year period during which the Hib and MenC vaccines were already included in our national vaccination program and a pneumococcal conjugate vaccine was commercially available. Similar studies have been performed in our country, although to the best of our knowledge, this is the first to include a period of time after the MenB vaccine became commercially available (2014) and after the introduction of the PCV-13 vaccine in our national vaccination program (2015).
We found 638 positive BC (6.6% of total BC), with 120 (1.2% of total BC) considered to represent true bacteremia. These rates are lower than those found in a previous study in our department (2003–2007), in which 14.4% of all BC were positive and 2.7% represented true bacteremia.16 Comparisons with other studies are limited by variations in criteria for BC collection, differences in patient population (e.g., proportion of patients with risk factors for bacteremia) and differences in time periods during which the studies were performed. Still, several studies performed between 2008 and 2013 have found rates of true bacteremia between 1.2% and 2.0%, similar to ours.6,7,12,17
Streptococcuspneumoniae was the most commonly identified microorganism, particularly in children aged three months or older. However, it should be noted that a significant proportion of cases occurred in 2008 (42.8%). Over the following years, the prevalence of pneumococcal bacteremia steadily decreased, with approximately one case per year between 2012 and 2015. In fact, between 2010 and 2015, Staphylococcus aureus was the most commonly isolated microorganism (except in 2012, when three cases of meningococcal bacteremia were registered). There was however an atypical occurrence of four cases of pneumococcal bacteremia in 2016. Whether this represents a true increase in incidence or a small fluctuation from baseline is difficult to determine, as data from after 2016 were not analyzed.
Previous studies in our country have found different trends after the first pneumococcal conjugate vaccines became commercially available and before PCV-13 introduction in our national vaccination program. In a nationwide study, the incidence of invasive pneumococcal disease decreased from 8.2/100,000 in 2008–2009 to 4.5/100,000 in 2011–2012.18 In a study by Garcez et al., there was also a steady decline in cases of pneumococcal bacteremia after 2006.1 On the other hand, in a study by Ferreira et al., no clear decline in the prevalence of pneumococcal invasive disease was found between 1995 and 2015.19 Interpretation of our results is however limited due to lack of information regarding serotypes. Future studies in our department may help clarify this finding and the impact of the introduction of the PCV-13 vaccine on our universal vaccination schedule.
Staphylococcus aureus was the second most commonly isolated microorganism both in the overall population and in those aged 36 months or older. The most common microorganism in children aged less than three months was Escherichia coli, the third most common agent in the overall population. This translates into the most commonly identified diagnosis in both age groups, as UTI was the most common diagnosis in those aged less than three months and osteoarticular infection was the second most common diagnosis in those aged 36 months or older.
Neisseria meningitidis accounted for ten cases of bacteremia. No serogroup C Neisseria meningitidis isolates were identified, although information was not available for four cases. Those with bacteremia due to serogroup B Neisseria meningitidis had not received any dose of the MenB vaccine. Haemophilus influenzae was identified in six cases; all had received at least two doses of the Hib vaccine. However, information regarding Haemophilus influenzae serotyping could not be retrieved, limiting interpretation.
All Streptococcuspneumoniae isolates were susceptible to penicillin. This in contrast with a previous study in our department in which 11% of Streptococcus pneumoniae isolates were resistant and 3.7% had intermediate susceptibility to penicillin.16 A more recent nation-wide study found an even higher prevalence of non-susceptibility to penicillin, with 20.9% of isolates expressing low-level resistance and 7.1% high-level resistance.18 The prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in our study was 21.7%; this is higher than what was found in previous studies in pediatric populations both in our department (8.3%) as well as in other centers in our country (0–8.7%).1,2,16 However, it is in accordance with data from the local Department of Clinical Pathology, where the prevalence of MRSA has remained close to 32% over the last few years (data not published). It is even lower than the last reported prevalence of MRSA in Portugal (47.4%) according to a report from the European Centre for Disease Prevention and Control (2011–2014).20 This may point to the need to revise local protocols of empirical antimicrobial therapy for infections in which Staphylococcus aureus is a common agent. We also found a higher incidence of Escherichia coli resistance to the association of amoxicillin and clavulanic acid (21.4%) compared with a previous study by Garcez et al. (6.3% of isolates were resistant and 12.5% had intermediate susceptibility).1 Of note, one isolate was resistant to second generation cephalosporins. Together, this reinforces the importance of knowing and periodically reviewing local antimicrobial susceptibility patterns.
Contaminants accounted for 5.4% of all BC and 81.2% of positive BC. This is lower than the contaminant rate found in a previous study in our department (11.7%), as well as that described by Garcez et al. (14.3%), while other studies have found rates between 2.0% and 3.9%.1,2,6,11,14,15 BC contamination is a significant problem, as it can lead to unnecessary hospital admissions, antibiotic therapy, and diagnostic tests.5,15 In an attempt to decrease child discomfort, most pediatric centers collect a single BC, and sometimes indwelling catheters are used for collection. Additionally, “opportunistic” BC are often obtained with initial blood work to decrease the need for a second venipuncture. Together, these may contribute to higher contamination rates in this population.1,21 In previous studies, BC contamination rates have also been inversely related with the volume of blood that is collected.15 Although the reason for this is unclear, it is possible that low volumes may result from difficult blood collections with decreased ability to maintain a sterile technique. It is also possible that with low blood volumes contaminant organisms may be present in higher concentrations.15
Determining what constitutes a contaminant versus a true pathogen can be challenging and is ultimately a clinical decision.5,8 Some criteria, such as longer time to positivity, lower presenting fever and lower white blood cells counts have been associated with a higher likelihood of contamination.10 For the purpose of this study, and as previously described in similar studies, we classified microorganisms classically considered to be contaminants as potentially pathogenic if they were identified more than once in the same episode or in patients with a known immunodeficiency or indwelling medical device.6,12–15 This resulted in the identification of seven cases of bacteremia due to CoNS, two of them in previously healthy infants (aged two and nine months) presenting with OB with two BC identifying the same microorganism (Staphylococcus hominis and Staphylococcus epidermidis, respectively). Only three patients received antibiotics, and all had favorable outcomes (data not shown). The role of CoNS in nosocomial sepsis in infants admitted to Neonatal Intensive Care Units has been extensively reported.22,23 Their pathogenic role in immunosuppressed patients and in those with indwelling medical devices is also recognized.13,14,24,25 However, distinguishing between contamination and true bacteremia due to CoNS remains a challenge, and an individual case-by-case approach is still the most appropriate.
Pneumonia was the most common diagnosis associated with bacteremia, including in those children aged three months or older. On the other hand, urinary tract infection was the most common diagnosis in those under three months of age. Osteoarticular infections were the second most common diagnosis in those aged 36 months or older. However, considerations regarding diagnostic yield of BC in each of these infections cannot be made, as the prevalence of negative BC according to diagnosis was not evaluated for the purpose of this study.
There are some limitations to our study. First, its retrospective design means that data was collected from clinical records not specifically designed to provide information for the defined variables. This may have led to inaccuracies and reporting bias. Additionally, some older records were only available on paper and unfortunately could not be retrieved. Second, it is a single-center study with a small sample size, limiting the extrapolation of our results as well as the drawing of definitive conclusions. Third, for the purpose of this study, information regarding the reason for BC collection was not collected. Information for patients with negative BC, such as age or diagnosis, was also not obtained. In the future, this may help to determine BC diagnostic yield in our population in the new pneumococcal conjugate vaccination era. Further studies will help clarify the impact of universal PCV-13 and MenB vaccination after their introduction in our national vaccination program.
Authorship statementAll authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Mariana Ferreira, Mafalda Santos, and Jorge Rodrigues. The first draft of the manuscript was written by Mariana Ferreira and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript that has been submitted.
Conflicts of interestNone to declare.
The authors would like to thank Dr. Margarida Farinha, head of the local Department of Clinical Pathology, for providing the list of blood cultures collected in our department during the study period.