In Spain, the use of pneumococcal conjugate vaccines (PCVs) has led to a decrease in the incidence of vaccine serotypes causing invasive and non-invasive disease in vaccinated and unvaccinated children and adults. Further, the coverage of most of the resistant serotypes by vaccines resulted in an overall decline in antibiotic resistance.
As an undesirable effect, there was an increase in the non-vaccine serotypes causing infection, especially serotypes 1, 7F and 19A after PCV7 and serotype 8 after PCV13 approval, this making the beneficial effect of vaccination less apparent.
The inclusion of PCVs in childhood vaccination schedules, its approval for use in healthy adults and the increasing number of serotypes covered by the vaccines in development are strong strategies in the fight against pneumococcal disease. Nonetheless, the epidemiology of Streptococcus pneumoniae infections must be still under surveillance to detect new changes, given the high capacity for recombination and adaptability of this always-surprising microorganism.
En España, el uso de las vacunas conjugadas neumocócicas (VCN) ha significado una disminución en la incidencia de los serotipos vacunales causantes de enfermedades invasivas y no invasivas, tanto en niños vacunados como en no vacunados y en adultos. Además, al estar la mayoría de los serotipos con resistencia antibiótica cubiertos por las vacunas, su uso se ha visto acompañado de una disminución global de la resistencia.
Como efecto no deseable se ha constatado un aumento de los serotipos no vacunales causantes de infección, especialmente de los serotipos 1, 7F y 19A después de la comercialización de la VCN7 y del serotipo 8 después de la autorización de VCN13, lo que ha hecho que el efecto beneficioso de la vacunación fuera menos aparente.
La inclusión de las VCN en los programas de vacunación infantil, su autorización para su uso en adultos sanos y el creciente número de serotipos cubiertos por las vacunas en desarrollo son estrategias importantes en la lucha contra la enfermedad neumocócica. Sin embargo, aún se debe vigilar la epidemiología de las infecciones por Streptococcus pneumoniae para detectar nuevos cambios como consecuencia de la alta capacidad de recombinación y adaptabilidad de este siempre sorprendente microorganismo.
The human nasopharynx is the natural habitat for Streptococcus pneumoniae and for many other resident bacteria, with which pneumococci can exchange genetic material in a continuous adaptive process.1 As a consequence, any activity that affects the nasopharyngeal microbiota, such as the use of antibiotics or vaccines, will directly influence the epidemiology of pneumococcal infection.2 In the nasopharynx, S. pneumoniae persists as a temporary colonizer especially in young children, it being transmissible from person to person through respiratory droplets in the air or direct contact with secretions from carriers. Once in the new host and depending on various factors, including immune status, prior contact with other pneumococci and the presence of concurrent respiratory viral infections, the pneumococcus may adhere to the respiratory epithelium, temporarily forming part of the respiratory microbiota. From there, it may spread causing a local (otitis, sinusitis, conjunctivitis, bronchitis, pneumonia, etc.) or systemic (sepsis, meningitis, etc.) infections.
The external polysaccharide capsule is the most important virulence factor of pneumococcus, although other factors are also implicated in its invasive capacity.3,4 Depending on the nature and arrangement of the sugars forming the capsule, a specific immune response will be elicited in the host. Since the pre-antibiotic era, it has been known that antibodies against the capsule protected against infection and serum therapy has been used for the treatment of severe pneumococcal infections.5 In fact, the study of the serum of convalescent patients led to the discovery of the different S. pneumoniae serotypes, more than 95 having been described to date.6,7 Although all serotypes are potentially capable of causing severe infection, some are considered “invasive” because they are more frequently detected in cases of invasive disease than in carriage, and a limited number of serotypes are responsible for most invasive infections.8 Further, other host-related factors such as underlying diseases and/or certain habits can increase the risk of developing invasive pneumococcal disease (IPD). For instance, the incidence of IPD in diabetic patients or in people who abuse alcohol is 5 and 11 times higher than in the general population, respectively.9 In some of these groups of patients, pneumococcal serotypes with low invasive potential are among the most frequent cause of IPD.
First vaccinesThe first pneumococcal vaccines marketed date back to 1909 and were composed of whole cells inactivated by heat, mimicking the vaccines for cholera, typhoid and plague.10 In the 1930s, the first vaccines composed of purified capsular polysaccharide, which only included serotypes 1 and 2, began to be used.11,12 At that time, antibiotics were not yet available for the effective treatment of bacterial infections and vaccination was recognized to be an important strategy in the fight against infections. The use of sulphonamides and especially of penicillin in the 1940s meant a dramatic change in the management of pneumococcal disease and a loss of interest in vaccines until 1967 when the first strains with low-level penicillin resistance appeared.13 Difficulties with treatment together with the emergence of multidrug-resistant strains14 renewed interest in the prevention of pneumococcal disease with capsular vaccines, despite the inconveniences of purified polysaccharide vaccines (PPVs), that did not confer long-term protection, required periodic revaccination and had no activity in children under 3 years old, an age group with a high incidence of IPD. Throughout the 1980s, new serotypes were progressively incorporated to PPVs until the 23-valent PPV (PPV23) reached the market. The PPV23 is still used today for adult immunization in some countries.10,15 Although the effectiveness of this vaccine in preventing pneumococcal pneumonia and IPD is around 50–60%, no significant changes in the serotype distribution have been linked with its use, probably because of its lack of effect on colonization and low vaccination rates. In Spain, the differences in vaccination schedules between the regional health systems mean that the PPV23 is not systematically used in all regions for adults, except in patients with some underlying diseases in which it is given as a booster after primary vaccination with the 13-valent pneumococcal conjugate vaccine (PCV).
Conjugate vaccinesThe first studies on conjugate vaccines, in which bacterial capsular polysaccharides are attached to a carrier protein to form a conjugate, were performed with pneumococcus polysaccharide and horse globulin or egg albumin in 1929.15 It was not until the end of the 1980s, however, that the first conjugate vaccines for the prevention of Haemophilusinfluenzae b (Hib) infections were licensed. In these new conjugate vaccine, the Hib polysaccharide was bound to a transporter protein (e.g., diphtheria or tetanus toxoid) changing the immune response against the capsular antigen from T-independent to T-dependent and the vaccine leading to the development of immunological memory (no revaccination being necessary) and being able to induce an immune response in children younger than 2 years of age.16 A few years later, following the process of Hib vaccine production, approval was given for conjugate vaccines against Neisseria meningitidis serogroups A and C, and in early 2000, the first conjugate vaccines against S. pneumoniae.17,18 Unlike Hib and N. meningitidis vaccines, the main problem when designing S. pneumoniae conjugate vaccines is the large number of different capsular types, it not being possible to include them all in a single formulation. Therefore, the first PCV included the capsular antigens of the 7 serotypes that most often caused invasive disease in children in Europe and the USA (serotypes 4, 6B, 9V, 14, 18C, 19F and 23F).19
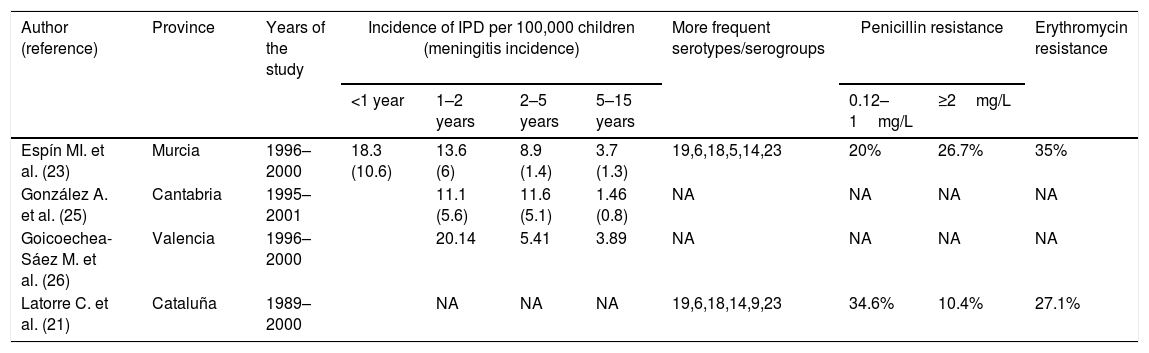
The first heptavalent PCV (PCV7) marketed in February 2000 in the USA (June 2001 in Spain) prompted the publication of several studies on the incidence of IPD prior to the use of the vaccine that have served as a baseline for subsequent studies on the efficacy of this vaccine.18 The incidence of IPD in under-2-year-olds varied from 11.1 cases per 100,000 in Cantabria to 76 cases per 100,000 in Barcelona (Table 1), the theoretical coverage of PCV7 being between 60.5% and 80.2% of cases.20–26 The incidence of meningitis in children under 2 years of age was less variable, ranging from 5.6 to 8.1 cases per 100,000 children.20,23,25 The percentage of pneumococcal isolates with high- and low-level resistance to penicillin was around 10% and 35% and more than 30% of invasive isolates were erythromycin resistant.21,23,24
Incidence of children's invasive pneumococcal disease (IPD) by age group, serotypes and antibiotic resistance rates in children in Spain before PCV7 introduction in 2001.
| Author (reference) | Province | Years of the study | Incidence of IPD per 100,000 children (meningitis incidence) | More frequent serotypes/serogroups | Penicillin resistance | Erythromycin resistance | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| <1 year | 1–2 years | 2–5 years | 5–15 years | 0.12–1mg/L | ≥2mg/L | |||||
| Espín MI. et al. (23) | Murcia | 1996–2000 | 18.3 (10.6) | 13.6 (6) | 8.9 (1.4) | 3.7 (1.3) | 19,6,18,5,14,23 | 20% | 26.7% | 35% |
| González A. et al. (25) | Cantabria | 1995–2001 | 11.1 (5.6) | 11.6 (5.1) | 1.46 (0.8) | NA | NA | NA | NA | |
| Goicoechea-Sáez M. et al. (26) | Valencia | 1996–2000 | 20.14 | 5.41 | 3.89 | NA | NA | NA | NA | |
| Latorre C. et al. (21) | Cataluña | 1989–2000 | NA | NA | NA | 19,6,18,14,9,23 | 34.6% | 10.4% | 27.1% | |
| Author (reference) | Province | Years of the study | Incidence of IPD per 100,000 children (meningitis incidence) | More frequent serotypes/serogroups | Penicillin resistance | Erythromycin resistance | |||
|---|---|---|---|---|---|---|---|---|---|
| <2 years | <5 years | <15 years | 0.12–1mg/L | ≥2mg/L | |||||
| Pineda V. et al. (24) | Barcelona | 1990–2000 | 76 | 45 | 16.6 | 6,14,18,19,1,5 | 31.7% | 8.6% | 32.4% |
| Iglesias Sánchez L. et al. (20) | Gipuzkoa | 1999–2001 | 48.4 (8.1) | 34.5 (3.5) | 12.6 (1.3) | 60.5% PCV7 | 35.4% | 38.7% | |
NA: not available.
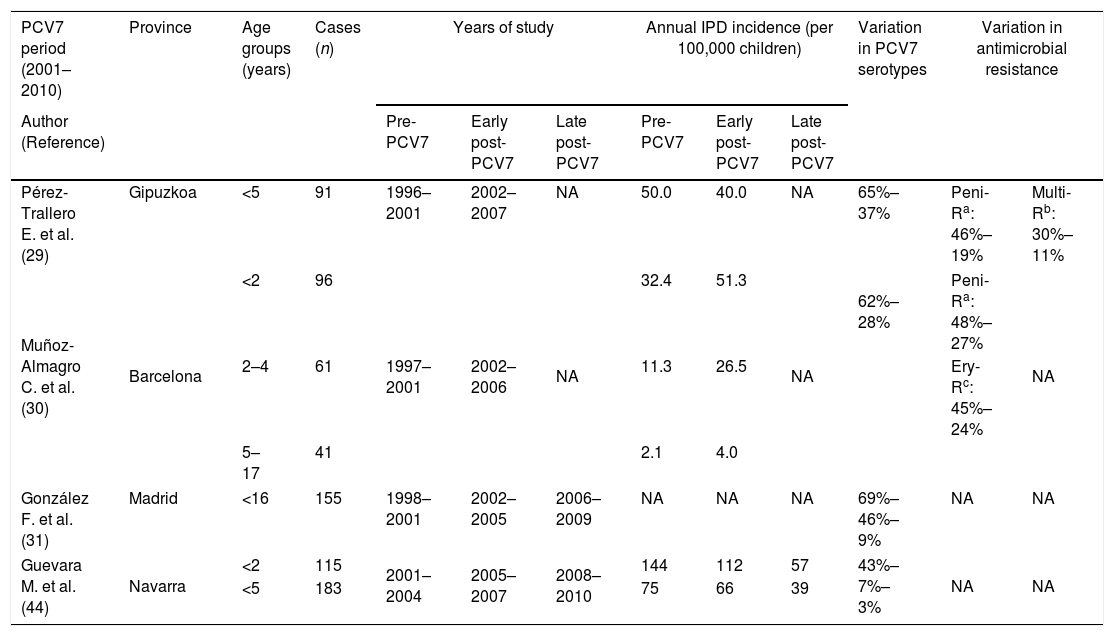
The administration of PCV7 was found to be safe, well-tolerated and immunogenic in children not previously vaccinated.27 Subsequent studies conducted in Spain in relation to PCV7 are difficult to compare since this vaccine was not included in the childhood vaccine schedule in most regions (the exception being Madrid, where it was included at the end of 2006) though its use at parents’ expense has progressively increased since its commercialization. Despite this variable vaccine use, that reached around 50% of infants in most regions, the same effects of decreases in IPD caused by vaccine serotypes and increases in non-vaccine serotypes were observed (Table 2). Nonetheless, the impact on the overall incidence of IPD has not been as apparent as in other countries, likely due to a different distribution of circulating serotypes. In Navarra, the effectiveness of PCV7 in preventing IPD due to vaccine serotypes was 88%.28 Notably, however, effectiveness decreased to 31% for overall IPD because vaccinated children had a 6 times higher risk of IPD caused by non-vaccine serotypes than unvaccinated children. Serotypes 19A, 1 and 3 were the most frequent among non-PCV7 serotypes. In Gipuzkoa, where the vaccination rate was estimated at 50%, there was no significant decrease in the overall incidence of IPD in children, though the incidence of IPD caused by vaccine serotypes fell by almost 60%.29 In addition, there was a decrease in resistance to penicillin (from 46% to 19%) and in multidrug resistance (from 30% to 11%), the serotypes included in the vaccine also being those which had shown the highest rates of antibiotic resistance.29 In Barcelona, an increase in the overall incidence of pediatric IPD was observed, with a slight decrease in PCV7 serotypes and an increase in the incidence of IPD caused by non-PCV7 serotypes, especially serotypes 1, 5, 6A and 19A.30 There was also a decrease in resistance to penicillin (from 48% to 27%) and to other antibiotics. In Madrid, the incidence of IPD due to non-PCV7 serotypes increased by 210% between 1998 and 2009.31 An increase in the incidence of pneumococcal pleural empyema was also observed, this being associated with an increase in the incidence of serotype 1.30,32
Incidence of pneumococcal disease, serotypes and antibiotic resistance rates in children in Spain after PCVs introduction.
| PCV7 period (2001–2010) | Province | Age groups (years) | Cases (n) | Years of study | Annual IPD incidence (per 100,000 children) | Variation in PCV7 serotypes | Variation in antimicrobial resistance | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (Reference) | Pre-PCV7 | Early post-PCV7 | Late post-PCV7 | Pre-PCV7 | Early post-PCV7 | Late post-PCV7 | ||||||
| Pérez-Trallero E. et al. (29) | Gipuzkoa | <5 | 91 | 1996–2001 | 2002–2007 | NA | 50.0 | 40.0 | NA | 65%–37% | Peni-Ra: 46%–19% | Multi-Rb: 30%–11% |
| Muñoz-Almagro C. et al. (30) | Barcelona | <2 | 96 | 1997–2001 | 2002–2006 | NA | 32.4 | 51.3 | NA | 62%–28% | Peni-Ra: 48%–27% | NA |
| 2–4 | 61 | 11.3 | 26.5 | Ery-Rc: 45%–24% | ||||||||
| 5–17 | 41 | 2.1 | 4.0 | |||||||||
| González F. et al. (31) | Madrid | <16 | 155 | 1998–2001 | 2002–2005 | 2006–2009 | NA | NA | NA | 69%–46%–9% | NA | NA |
| Guevara M. et al. (44) | Navarra | <2 | 115 | 2001–2004 | 2005–2007 | 2008–2010 | 144 | 112 | 57 | 43%–7%–3% | NA | NA |
| <5 | 183 | 75 | 66 | 39 | ||||||||
| PCV13 period (>2010) | Province | Age (years) | Cases (n) | Years of study | Annual IPD incidence (per 100,000 children) | Variation in PCV13 serotypes | Variation in antimicrobial resistance | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Author (Reference) | Pre-PCV13 | Post-PCV13 | Pre-PCV13 | Post-PCV13 | ||||||
| Santana Hernández M. et al. (43) | Gran Canaria | <14 | 190 | 2001–2010 | 2010–2016 | 13.1 | 4.4 | 77%–41% | Peni-Ra: 41%–5.3% | |
| Ery-Rb: 57%–8% | ||||||||||
| Guevara M. et al. (44) | Navarra | <2 | 35 | 2008–2010 | 2011–2014 | 57 | 36 | 75%–35% | NA | NA |
| <5 | 59 | 39 | 18 | |||||||
| Picazo JJ. et al. (45) | Madrid | <5 | 2007–2008 | 2015–2016 | 35.1 | 12.5 | 91% reduction | Peni-Ra: 54%–28.2%d | NA | |
| <15 | 912 | 2007–2008 | 2015–2016 | 17.1 | 5.1 | Ery-Rc: 31%–24%e | ||||
| Ciancoti LR. et al. (46) | Valencia | <5 | NA | 2007 | 2012 | 30.5 | 12.3 | 68%–44% | NA | NA |
| 5–9 | NA | 2007 | 2012 | 7.8 | 2.8 | NA | ||||
NA: not available.
The introduction of PCV7 was also followed by a decrease in non-invasive pneumococcal disease. Of the strains isolated from otitis received at the Spanish Pneumococcal Reference Laboratory, a decrease was observed in PCV7 serotypes from 70.7% in the pre-vaccine period (1997–2000) to 10.6% in 2009.33 In turn, an increase was observed in the rate of otitis caused by serotypes 19A, 3 and others not included in PCV7.32 In Gipuzkoa, there was a decline in the prevalence of otitis caused by PCV7 serotypes from 62.4% in the pre-PCV7 period to 2.4% in 2010, together with an increase in infections by serotype 19A, especially by the multidrug-resistant clones ST276 and ST320.34 In Barcelona, similar decreases were observed in the prevalence of otitis caused by vaccine serotypes, from 66% in 1992–2001 (pre-vaccine period) to 13% in 2007–2011.35 A very significant increase in otitis caused by serotype 19A ST320 was also observed.35 Serotype 19F did not decrease as much as might have been expected in ear infections, probably due to its strong tendency to cause otitis.35,36 A similar trend was observed in other pneumococcal infections including conjunctivitis, for which there was a decrease in infections caused by vaccine serotypes from 35% to 3% between 1999–2001 and 2008–2010, although pneumococcal conjunctivitis was mostly caused by unencapsulated strains.37
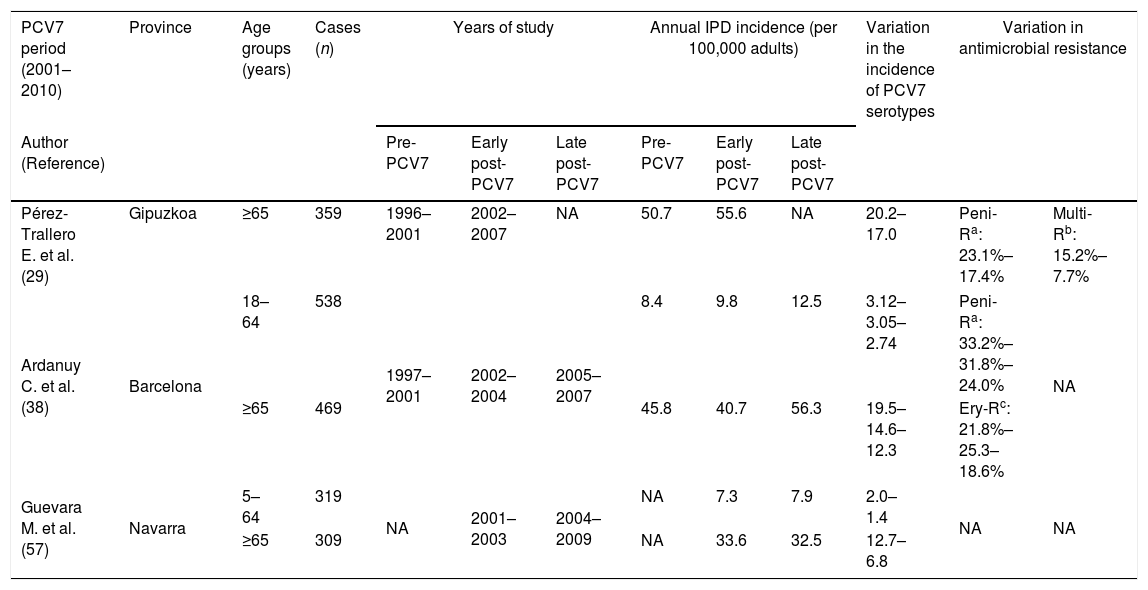
Indirect effects of child PCV7 administration in adultsDespite the low vaccination coverage in children, the introduction of PCV7 also had an effect on pneumococcal diseases in adults. The progressive eradication of pneumococcal PCV7 serotypes from children's nasopharynx was accompanied by a progressive decrease in pneumococcal diseases caused by these serotypes in the adult population, indicating a herd protection effect (Table 3).
Incidence of pneumococcal diseases, variation in serotype distribution and antibiotic resistance rates in adult population in Spain after PCVs introduction.
| PCV7 period (2001–2010) | Province | Age groups (years) | Cases (n) | Years of study | Annual IPD incidence (per 100,000 adults) | Variation in the incidence of PCV7 serotypes | Variation in antimicrobial resistance | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (Reference) | Pre-PCV7 | Early post-PCV7 | Late post-PCV7 | Pre-PCV7 | Early post-PCV7 | Late post-PCV7 | ||||||
| Pérez-Trallero E. et al. (29) | Gipuzkoa | ≥65 | 359 | 1996–2001 | 2002–2007 | NA | 50.7 | 55.6 | NA | 20.2–17.0 | Peni-Ra: 23.1%–17.4% | Multi-Rb: 15.2%–7.7% |
| Ardanuy C. et al. (38) | Barcelona | 18–64 | 538 | 1997–2001 | 2002–2004 | 2005–2007 | 8.4 | 9.8 | 12.5 | 3.12–3.05–2.74 | Peni-Ra: 33.2%–31.8%–24.0% | NA |
| ≥65 | 469 | 45.8 | 40.7 | 56.3 | 19.5–14.6–12.3 | Ery-Rc: 21.8%–25.3–18.6% | ||||||
| Guevara M. et al. (57) | Navarra | 5–64 | 319 | NA | 2001–2003 | 2004–2009 | NA | 7.3 | 7.9 | 2.0–1.4 | NA | NA |
| ≥65 | 309 | NA | 33.6 | 32.5 | 12.7–6.8 | |||||||
| PCV13 period (>2010) | Province | Age groups (years) | Cases (n) | Years of study | Annual IPD incidence (per 100,000 adults) | Variation in the incidence of PCV13 serotypes | Variation in antimicrobial resistance | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (Reference) | Pre-PCV13 | Early post-PCV13 | Late post-PCV13 | Pre-PCV13 | Early post-PCV13 | Late post-PCV13 | ||||||
| Cámara J. et al. (54)González-Díaz A. et al. (55) | Basque Country, Catalonia, Madrid | 18–50 | 570 | 2008–2009 | 2012–2013 | 2015–2016 | 7.1 | 3.0 | 2.6 | 4.4–1.3–0.7 | Peni-Ra: 22.7%–26.8%–21.2% | NA |
| 51–64 | 557 | 14.7 | 10.3 | 10.9 | 9.7–4.8–3.4 | |||||||
| 65–74 | 428 | 20.2 | 15.6 | 15.8 | 12.5–5.8–4.6 | |||||||
| ≥75 | 642 | 29.5 | 22.4 | 23 | 17.8–9.8–6.3 | |||||||
| Guevara M. et al. (57) | Navarra | 5–64 | 325 | 2004–2009 | 2010–2013 | NA | 7.9 | 5.2 | NA | 5.9–3.1 | NA | NA |
| ≥65 | 320 | 32.5 | 25.0 | NA | 22.6–12.9 | |||||||
| Latasa P. et al. (51) | Madrid | 15–39 | 446 | 2008–2010 | 2011–2012 | 2013–2015 | 3.6 | 2.0 | 1.5 | 2.2–0.8–0.5 | NA | NA |
| 40–59 | 1059 | 9.6 | 5.7 | 6.0 | 4.5–2.3–1.3 | |||||||
| >59 | 1811 | 18.0 | 16.5 | 17.1 | 9.4–5.5–4.7 | |||||||
NA: not available.
The impact of child PCV7 vaccination on IPD in adults began to be observed a few years after the vaccine came onto the market. Specifically, in the area of Barcelona in a late vaccination period (2005–2007) with an estimated vaccination rate greater than 50%, the rate of IPD due to PCV7 serotypes decreased by 23%; however, there was an 81% rise in the IPD caused by non-vaccine serotypes. This increase was associated with the emergence or dissemination of the so-called epidemic serotypes (1, 5, 7F) leading to a general increase in IPD in adults, especially in young adults.38 In the same period, no significant changes in the incidence of IPD among over-64-year-olds were observed in Gipuzkoa.29
As occurred in children,32 the spread of serotype 1 was associated with a rise in pneumonia with empyema in young adults (18–50 years of age), the rate nearly doubling among pneumonia episodes overall.39 Moreover, another study performed in adult IPD in Barcelona documented a significant increase in rates of septic shock (19.1% vs. 31.1%) after PCV7 introduction (1996–2001 vs. 2005–2009). This increase in septic shock was mainly linked to IPD due to serotypes 3 or 19A.40
The increase in serotypes 1, 5, and 7F, commonly susceptible to most antimicrobials, together with the drop in the prevalence of serotypes associated with multidrug resistance (23F, 6B, 19F), caused an overall decrease in resistance and multidrug resistance rates of S. pneumoniae in Spain.29,38,41,42 Nonetheless, the increase in some serotypes such as 19A and 24F, and especially of some multidrug-resistant lineages of these serotypes as ST276 and ST320 (19A) and ST230 (24F), partially counterbalanced this effect.38 This increased incidence of infections caused by non-PCV7 serotypes, especially 19A and to lesser extent serotypes 1 and 3 in both invasive and non-invasive infections, was seen as a risk that could reduce the benefit of vaccination programs.
Effects of high-valence pneumococcal conjugate vaccine administration in childrenThe approval in May 2009 of the 10-valent PCV (PCV10), which added serotypes 1, 5, and 7F to PCV7 serotypes, and in December 2009 of the 13-valent PCV (PCV13), which added serotypes 1, 3, 5, 6A, 7F, and 19A was accompanied by a general decrease in the incidence of invasive and non-invasive pneumococcal infections (Table 2).
In the Canary Islands, there was a 60.1% decrease in the incidence of IPD due to PCV13 serotypes in vaccinated children (from 13.1 to 4.4 cases per 100,000 children between 2001 and 2010).43 A similar situation was observed in other places such as Navarra, with reductions in pediatric IPD from 39 cases per 100,000 children in the period 2008–2010 to 18 cases in 2011–2014, with an overall 90% reduction in the rate of IPD caused by all serotypes and 95% vaccine effectiveness in IPD due to PCV13 serotypes.44 In a study carried out in Madrid, where PCV7 was replaced by PCV13 in the regional immunization program from June 2010 until May 2012, the overall reduction in IPD in children under 15 years old was 70.1% (from 17.1 cases per 100,000 children in 2007–08 to 5.1 cases in 2015–16) and 91% for IPD caused by PCV13 serotypes, especially serotypes 1, 5, 7F and 19A.45 In another study from Valencia, a decrease in the incidence of IPD was observed between 2007 and 2012, from 30.5 to 12.3 cases per 100,000 children younger than 5 years old, with no significant variation in the number of infections caused by non-vaccine serotypes.46 However, PCV13 did not demonstrate significant effectiveness in preventing IPD caused by serotype 3 compared to that observed for the other serotypes included in the vaccine.45,47 A herd protection effect was also observed in unvaccinated children, which showed a fall in the incidence of IPD due to vaccine serotypes of a similar magnitude to that seen in vaccinated children.44
PCV13 introduction was also accompanied by a decrease in the incidence of pneumococcal exudate or pleural effusion in children, related to the drop in the infections caused by serotype 1.45,48 A decline was also observed in the incidence of pneumococcal meningitis49 and hospitalizations due to pneumococcal infections, especially due to pneumonia in children younger than 5 years of age.50 It has also to be highlighted the decrease observed in pneumococcal antibiotic resistance, mainly due to a reduction in IPD caused by serotype 19A, since many invasive serotype 19A clones were resistant.45
In the pediatric population, there has been a slight emergence of non-PCV13 serotypes, especially serotypes 15B and 24F.51 Nonetheless, at present, the replacement effect does not seem to be as pronounced as that observed after the introduction of PCV7 with an overall decrease in IPD.44,46,52
The introduction of PCV13 resulted in an even greater decrease in non-invasive pneumococcal infections caused by vaccine serotypes that was also mainly due to a decline in infections by serotype 19A, which after the introduction of PCV7 had become the most prevalent otitis-causing serotype.33–35 In a study carried out in Barcelona and Gipuzkoa, it was observed that pneumococcal otitis decreased after the introduction of PCV13, mainly due to a decrease in disease caused by vaccine serotypes, except for serotype 3 in Gipuzkoa and 19F in both regions.53
Indirect effects of child PCV13 vaccination in adultsThe replacement of PCV7 with PCV13 occurred in a general scenario of higher childhood vaccination rates, which had a correspondingly larger impact on adult pneumococcal disease (Table 3). A multicenter study in six Spanish hospitals in Catalonia, Gipuzkoa and Madrid demonstrated a significant reduction in the overall adult IPD rate 4 years after the introduction of PCV13 (2008–09 vs. 2012–13) in both younger (18–64 years old) and older adults (≥65 years old).54 This was associated with a significant decrease in IPD caused by PCV13 serotypes. This herd protection effect was most marked in Madrid, where PCV13 had been introduced in the official childhood vaccination schedule. A second analysis of this same study showed a stabilization of IPD in adults in the late-PCV13 period (2015–16). In this latter period, the continuous decrease in IPD caused by PCV13 was counterbalanced by an increase in serotypes not included in PCV13. Among these, serotype 8 showed the most significant increase.55 This multicenter study indicated that, like in children, the rate of IPD caused by serotype 3 (included in PCV13) did not decrease in adults.45,47 Further, a study carried out in Madrid also showed a progressive and significant decline in IPD associated with a decrease in PCV13 serotypes in adults between 18 and 59 years old. This was again associated with an increase in non-PCV13 serotypes and especially in serotype 8.51 In Gran Canaria, a 66.4% decrease in IPD in all age groups was observed after the introduction of the PCV13 and prior to it being included on the official vaccination schedule (2001–2016).39 Likewise, a study performed in Mallorca analyzing pneumococcal infections in adults showed a decrease in PCV13 serotypes as a cause of bacteremic and non-bacteremic pneumococcal pneumonia.56 In Navarra, in the PCV7 period (2001–2009), while rates of IPD had remained stable in adults, significant reductions were observed among 5- to 64-year-olds (34%) and older adults (over 64 years) (23%) 3 years after PCV13 introduction.57 This was linked to a progressive increase in the rates of vaccinated children (total or partial) up to 78% in 2013. Furthermore, an overall increase was observed in the percentage of patients with IPD and underlying diseases (64% in 2004–2009 to 75% in 2010–2013) or immunocompromised (14%–25%).57
Herd protection in adults due to child PCV13 vaccination has also been seen in other European countries,58 with an overall decline in IPD rates. Nonetheless, as discussed above, the increase in non-PCV13 serotypes, especially in serotype 8, largely counterbalanced this decrease. Surprisingly, a rise in serotype 8 IPD has not been observed in the USA, as it was in the 2000s with epidemic serotypes (1, 5, 7F).38,59 Various factors could explain this, such as differences in sampling strategies between sites, transmission models, circulating pneumococcal clones or risk factors.59
In adult patients, pneumococci are frequently associated with acute exacerbation of chronic obstructive pulmonary disease (AECOPD).60 Two recent studies analyzed the impact of child PCV13 vaccination in pneumococcal infections in COPD patients showing an overall decrease in PCV13 serotypes among AECOPD isolates (55.2% in 2001–04 to 16.2% in 2013–2016).60,61 A worrisome finding of this study was an increase in beta-lactam resistance among serotype 11A isolates. This increase was linked to a clonal shift in this serotype. The emerging clone CC156-11A was previously associated with serotypes included in the PCV7 vaccine (9V and 14). This vaccine escape recombinant clone has been also detected as a cause of adult IPD.54,55
More promisingly, childhood vaccination has had an impact on mortality in adult patients with IPD. In adults aged 18 to 64 years, IPD mortality decreased from 22% (1994–2001) to 14% (2002–09) and then to 12% (2010–2013).62 This decrease was mainly observed among cases of bacteremic pneumonia and was linked to a reduction in mortality caused by PCV7 serotypes. In older adults, although mortality rates due to PCV7 serotypes declined over time, the overall rate remained stable, probably due to other age-related factors.62 Furthermore, a national study covering 98% of public hospitals showed a significant decline in the hospitalization rates among ≥65-year-olds, mainly due to a decrease in pneumococcal pneumonia.50
ConclusionsIn children, vaccination with pneumococcal conjugate vaccines has led to a progressive decrease in IPD in both vaccinated and unvaccinated populations. The increase in the incidence of non-vaccine serotypes after 19 years of use of conjugate vaccines is currently much less than the decrease in the incidence of IPD caused by vaccine serotypes, which has undoubtedly resulted in a very important net benefit for the pediatric population. Further, a marked decrease has been observed in non-invasive infections and in antibiotic-resistant isolates in children.
On the other hand, in the adult population, especially among individuals over 65 years of age, the benefit has been counterbalanced by an increase in serotypes not included in conjugate vaccines. The recent introduction of conjugate vaccines in the strategy for the prevention of IPD in adults in some autonomous regions in Spain (Madrid, Castilla-León, La Rioja, and Galicia) opens a new chapter in the follow-up of IPD in adults.
The development of new conjugate vaccines including more serotypes nearing market release gives new hope in the fight against pneumococcal diseases.
Conflict of interestsJose Maria Marimon and Carmen Ardanuy have been members of an Advisory Board for PCV13 for Pfizer.