Urinary tract infections (UTIs) are a common problem in the elderly population. Urine culture is still considered the “gold standard” to diagnose infection in this population. However, urine cultures are laborious and costly, and most samples will yield no growth.
MethodsAn evaluation was made of the Sysmex UF-1000i flow cytometer as a screening tool for UTI in an elderly population older than 65 years who lived in the community, using 346 urine samples submitted for culture.
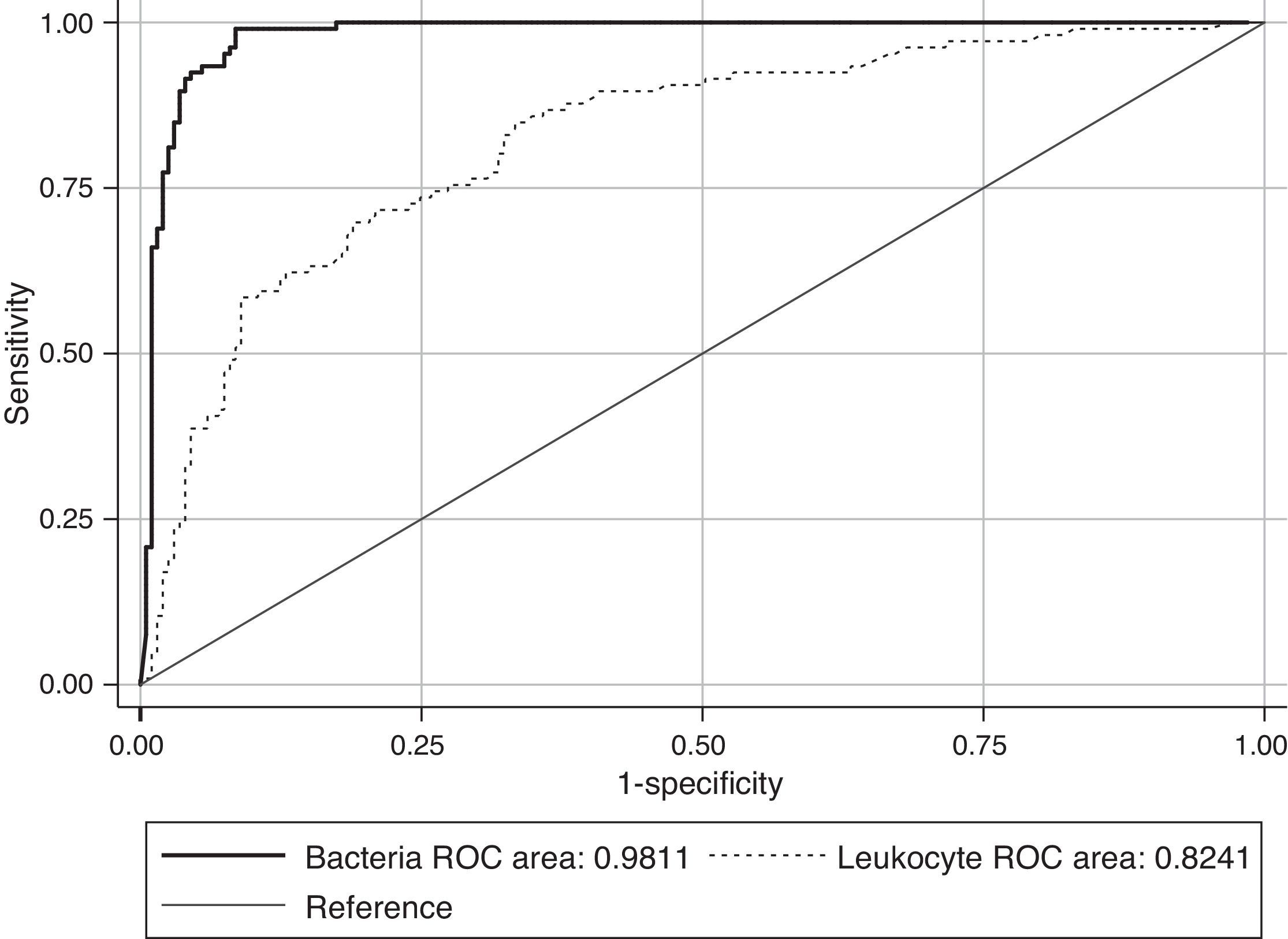
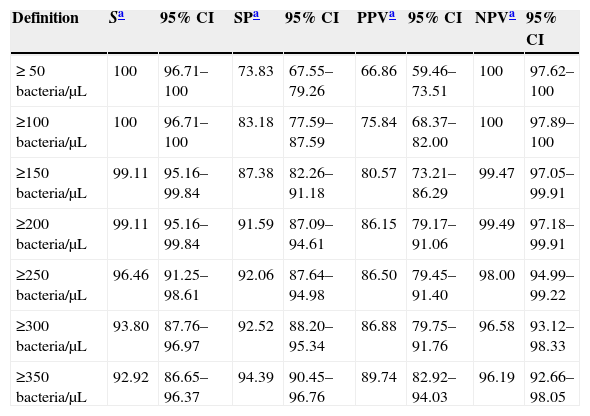
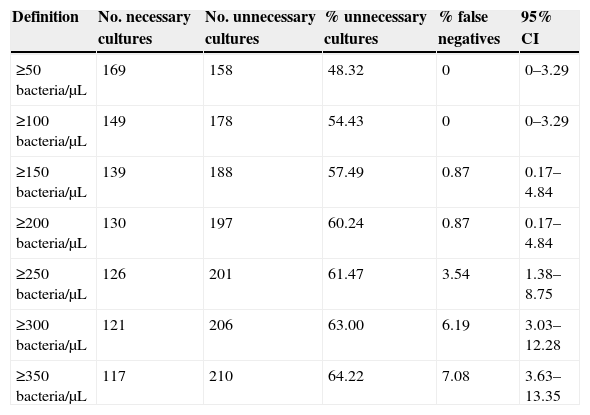
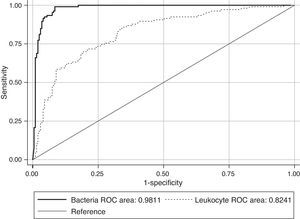
ResultsThe Receiver Operating Characteristic (ROC) analysis showed a significant difference (P<0.01) between 0.98 bacteria area under the curve value and 0.82 of white blood cells (WBC). The combination of both counts for screening did not show any improvement in specificity or sensitivity. According to our data, the use of a single cut-off point of 200bacteria/μL is suggested, in which the sensitivity and specificity were 99.11% and 91.59%, respectively, with a NPV of 99.49%. Moreover, this cut-off value could avoid 60.24% of the samples to be cultured, with a minimal false negative results rate of 0.87%.
ConclusionsThe stratification of age groups stratification helps in selecting a more adjusted Sysmex UF1000i cut-off limit, leading to an improvement in the screening parameters that would imply a better management of these infections, as well as a high reduction in the workload and cost savings.
Evaluar y optimizar el uso del citómetro de flujo (Sysmex UF1000i®) como cribado para las infecciones urinarias (ITUs) en pacientes ≥65 años procedentes de Atención Primaria.
MétodosSe estudiaron 346 orinas de pacientes ≥65 años con sospecha de infección urinaria, enviadas al Hospital Universitario Virgen del Rocío, durante el periodo enero-mayo 2013. Las muestras se estudiaron mediante citometría de flujo y cultivo cuantitativo en medio cromogénico.
ResultadosSe incluyeron 346 pacientes, cuya edad media fue de 76,70±0,75 años. De las 346 muestras 113 (32,65%) fueron positivas, 214 (61,84%) negativas y 19 (5,49%) contaminadas. El área bajo la curva ROC utilizando el número de bacterias (0,98) fue mayor que para los leucocitos (0,82), existiendo diferencias significativas entre ellas (P<0.01). El estudio conjunto de bacterias y leucocitos no supuso ninguna mejora, por lo que se utilizaron distintos umbrales basados en el número de bacterias. De acuerdo con nuestros datos, proponemos un punto de corte de 200 bacterias/μl con el cual obtenemos una sensibilidad del 99,11% y especificidad del 91,59%, con un valour predictivo negativo del 99,49%. Además este punto de corte nos permitiría evitar el 60,24% de los cultivos, con una mínima tasa de falsos negativos (0,87%).
ConclusionesLa categorización de la población según criterios de positividad para el diagnóstico de la ITU, así como en grupos etarios con las mismas condiciones clínicas, permite incrementar la exactitud de los resultados obtenidos, reduciendo la carga de trabajo sustancialmente así como los costes asociados.
In the elderly population urinary tract infections (UTIs) are common, representing the second most frequent infection in elderly women living in the community.1,2 The diagnosis of UTI requires the presence of significant bacteriuria (≥105 Colony Forming Unit (CFU) per mL) and genitourinary symptoms. However, these patients may not refer typical urinary complaints; thus, the diagnosis may be more challenging than in other age groups. Most of these cases are self-limited and have no long-term sequelae, but underlying structural or functional abnormalities of the genitourinary tract are not rare,3 and serious complications may develop, such as pyelonephritis or sepsis.4,5
Microbiologic cultures are still considered the diagnostic “gold standard”, allowing identification and quantification of the causal agents.6 Nevertheless, culturing of the samples is both time and labour consuming, with most of the samples yielding no growth.3,7,8 Screening methods can improve the laboratory efficiency by ruling out UTI-negative samples, thereby reducing the workload. The Sysmex UF-1000i (TOA Medical Electronics, Kobe, Japan) is an automated urine particle analyzer using laser-based fluorescent flow cytometry. Some data have shown that it is valid as a screening test for UTIs, suggesting that cut-off values should vary depending on both age and gender.9–11 Further studies are required to establish the value of this system, interpret the results and adapt the criteria of a positive result to the characteristics of a given population.
In 2012, 34,128 urine samples from Primary Care Units were received at the Microbiology Service of the Virgen del Rocío University Hospital, out of which 10,223 (29.95%) were from elderly patients, 70% being negative cultures (data not shown). This high number of negative specimens emphasizes the need for a urine samples screening method prior to be cultured, and therefore to improve the laboratory efficiency by reducing not only the economical and workload costs but also by shortening the laboratory response time.
The aim of this study was to evaluate and to optimize the use of the Sysmex UF-1000i as a screening method for urine samples obtained from an in community-dwelling elderly population older than 65 year.
Materials and methodsPatients and urine samplesFrom January 2013 to June 2013, 346 patients attending at the Primary Care Units of Virgen del Rocío University Hospital were selected using a systematic random sampling. Four to five urine samples per day submitted for culture from elderly outpatients (≥65 years old) were randomly selected. Sample size was determined by the Carley et al. method,12 using a 95% of sensitivity and a precision of 5% for the expected UTI prevalence of elderly patients. Midstream catch urine was collected in sterile preservative tubes SRO-1-25B with boric acid (Soria Melguizo S.A., Madrid, Spain), transported at cold temperature and processed within 4–8h after the sampling.
Culture and urinalysisTen microlitres of the urine specimen were quantitatively cultured onto Brilliance UTI Clarity Agar (Oxoid, Basingstoke, UK) plates. All plates were aerobically incubated for 18–24h at 37°C, and the results were expressed as the number of colony-forming units (CFUs) per millilitre. A threshold of ≥105CFUs/mL for women and ≥104CFUs/mL for men was established for positive cultures. The presence of two or more different isolates as well as the growth of one or more non-pathogens was defined as contamination of the specimen. Identification of the isolates was performed by conventional biochemical tests (biochemical testing, pigment production, growth, and colony characteristics) and MicroScan WalkAway®plus System (Siemens Healthcare Diagnostics, WestSacramento, CA). When the identification was uncertain, it was confirmed by Bruker Biotyper MALDI-TOF MS system (Bruker Daltonik GmbH, Leipzig, Germany).
All of the urine specimens were also analyzed by the Sysmex UF-1000i, which allows the discrimination and quantification of bacteria, erythrocytes, WBC, epithelial cells, casts, crystals, fungi, and sperm. The results obtained with Sysmex UF-1000i and those from the urine cultures were compared.
StatisticsA logistic regression model was performed, in which age, gender, bacterial count and WBC count were included as independent variables, to predict the probability of a positive culture. The cut-off values for bacteria and leucocytes were evaluated in the Sysmex UF-1000i according to the area under the ROC curve, which was estimated by using the Hanley and McNeil's nonparametric method.13 The bacteria and leucocytes ROC curves were compared by the De Long et al. method.14 Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for those cut-off points. A P value ≤0.05 was considered as significant. The data analysis was carried out using the software STATA Release 10.1 statistical package (StataCorp LP, Lakeway Drive, TX, USA).
ResultsIn total, 346 elderly patients, 261 women (75, 43%) and 85 men (24, 56%) were included in this study. The mean age was 76.70±0.75 years. One hundred and thirteen (32.65%) of the urine samples yielded positive cultures, 214 (61.84%) were negative and 19 (5.49%) were considered as contaminated and not further tested. The clinical isolates obtained were: Escherichia coli (72), Klebsiella pneumoniae,13Proteus mirabilis,9Enterococcus faecalis,5Pseudomonas aeruginosa,4 ESBL-E. coli,3 ESBL-K. pneumoniae,1Klebsiella oxytoca,1Serratia liquefaciens,1Morganella morgani,1Citrobacter freundii,1Citrobacter koseri1 and Citrobacter amalonaticus.1
The ROC curves for WBC and bacterial counts from the UF-1000i are given in Fig. 1. The area under the curve (AUC) for bacterial counts was 0.98, a significantly higher value than the 0.82 AUC for WBC count (P<0.01). WBC count was not as effective as the former as screening method for UTI, and the use of both bacterial and WBC counts did not show any advantage in terms of sensitivity or specificity compared with bacterial counts alone. Therefore, only different cut-off values for bacterial counts have been applied to predict community-acquired UTI in the elderly. Parameters associated with the different cut-off values for bacterial counts generated by Sysmex UF-1000i are shown in Table 1.
Parameters depending on the cut-off values for the bacterial count.
| Definition | Sa | 95% CI | SPa | 95% CI | PPVa | 95% CI | NPVa | 95% CI |
|---|---|---|---|---|---|---|---|---|
| ≥ 50bacteria/μL | 100 | 96.71–100 | 73.83 | 67.55–79.26 | 66.86 | 59.46–73.51 | 100 | 97.62–100 |
| ≥100bacteria/μL | 100 | 96.71–100 | 83.18 | 77.59–87.59 | 75.84 | 68.37–82.00 | 100 | 97.89–100 |
| ≥150bacteria/μL | 99.11 | 95.16–99.84 | 87.38 | 82.26–91.18 | 80.57 | 73.21–86.29 | 99.47 | 97.05–99.91 |
| ≥200bacteria/μL | 99.11 | 95.16–99.84 | 91.59 | 87.09–94.61 | 86.15 | 79.17–91.06 | 99.49 | 97.18–99.91 |
| ≥250bacteria/μL | 96.46 | 91.25–98.61 | 92.06 | 87.64–94.98 | 86.50 | 79.45–91.40 | 98.00 | 94.99–99.22 |
| ≥300bacteria/μL | 93.80 | 87.76–96.97 | 92.52 | 88.20–95.34 | 86.88 | 79.75–91.76 | 96.58 | 93.12–98.33 |
| ≥350bacteria/μL | 92.92 | 86.65–96.37 | 94.39 | 90.45–96.76 | 89.74 | 82.92–94.03 | 96.19 | 92.66–98.05 |
The most balanced cut-off value was 338bacteria/μL, with sensitivity, specificity and negative predictive (NP) values of 93.81%, 93.93% and 96.39% respectively. Nevertheless, screening for negative urine samples requires both a high sensitivity and high NP values in order to minimize the number of false-negative results. Sensitivity and specificity values of 99.11% and 91.59% respectively, with a NPV of 99.49% (Table 2) were obtained with a cut-off of 200bacteria/μL.
Number and percentage of savings with different cut-off values.
| Definition | No. necessary cultures | No. unnecessary cultures | % unnecessary cultures | % false negatives | 95% CI |
|---|---|---|---|---|---|
| ≥50bacteria/μL | 169 | 158 | 48.32 | 0 | 0–3.29 |
| ≥100bacteria/μL | 149 | 178 | 54.43 | 0 | 0–3.29 |
| ≥150bacteria/μL | 139 | 188 | 57.49 | 0.87 | 0.17–4.84 |
| ≥200bacteria/μL | 130 | 197 | 60.24 | 0.87 | 0.17–4.84 |
| ≥250bacteria/μL | 126 | 201 | 61.47 | 3.54 | 1.38–8.75 |
| ≥300bacteria/μL | 121 | 206 | 63.00 | 6.19 | 3.03–12.28 |
| ≥350bacteria/μL | 117 | 210 | 64.22 | 7.08 | 3.63–13.35 |
Several studies, with a considerable heterogeneity in variables such as gender, age or clinical condition (in or outpatients, comorbidities, use of medical devices, etc) have shown the usefulness of the UF-1000i screening for UTI.4,6,9 The use of a unique standard cut-off value for a heterogeneous population results in over- or under-culturing specimens among different groups, thereby affecting both predictive values and diminishing the reliability of the screening. In addition, stratification according to different established UFC/mL criteria for the diagnosis of UTI is likely to increase the accuracy of the studies’ results in different groups of populations.15 These and other factors should be also considered when deciding the proper cut-off value in a precise group of population. Regarding elderly people, the criteria of 105UFC/mL for women and 104UFC/mL for men meet the standard one, but the higher prevalence of UTI increases the PPV and decreases the NPV. Moreover, misclassification of patients with or without UTI may have serious implications due to frequent presence of multimorbidity, polytreatment and impaired renal function in this age group. All these factors emphasize the convenience to lower the ROC curve most balanced cut-off value in order to raise both sensitivity and NPV, and therefore to achieve a lower false negative rate. In addition, fast and reliable negative results may allow an antibiotic treatment discontinuation, bringing along a decrease in the risk of drug interactions and side effects in an often-multimorbid patient.
In our study, the sensitivity, specificity, and AUC of bacterial count in the Sysmex UF-1000i analyzer system were higher than those of WBC count, and the combination of both counts for screening did not show any specificity or sensitivity improvements to bacterial counts alone, as previously reported.7 Nevertheless, in contrast with these results, some articles showed the effectiveness of screening with WBC plus bacterial counts,6,10 with an increase in sensitivity but a decrease in specificity. This difference could be attributable to the two different populations studied, outpatients only7 or both in- and outpatients.6,10 Moreover, some studies reveal that pyuria is not a good marker of UTI. In the study by Kishore et al., the relationship between pyuria and culture positivity did not reach statistical significance in both males and females in the community elderly.16 Another study performed by Kupelian et al.17 reported that pyuria demonstrates poor sensitivity in patients with UTI. A systematic review and meta-analysis performed by Shang et al. conclude that UF-1000i may be used as an effective screening method for UTI by measuring WBC and bacterial counts of urine samples.18 Nevertheless, in this study the overall estimates of sensitivity and specificity show a very high heterogeneity (i2>90%), limiting the validity of these results. Broeren et al.3 hypothesized that the lack of improvement shown in some studies was due to the lack of use of boric acid, which acts as a stabilizing compound. Kupelian et al.17 describes that cell destruction appears to be retarded by boric acid, although significant cell loss appears inevitable. Our data do not support this hypothesis, as we used boric acid containers and no improvement was observed. It has been suggested that gender-specific cut-off points could improve the screening,10 but again we did not find any significant differences between men and women for bacterial counts.
According to our data, the recommended cut-off value is 200bacteria/μL, higher than those mentioned in previous studies,6,7,19–22 and with which, we obtain a 60, 24% reduction of the samples to be cultured, with a very low 0.87% false negative rate. The only false negative culture result found was a>105CFU/mL Proteus mirabilis culture. False negative results mainly with Gram-positive pathogens,14,17 but also with Gram-negatives have been documented with the use of Sysmex UF-1000i.23,24 Further studies using larger sample sizes would probably permit to address this issue. A limitation in the design of our study is that the cohort was selected from urine samples sent to the laboratory instead of from a group of patients eligible for UTI.
ConclusionsIn conclusion, age-groups stratification allows selecting a more adjusted Sysmex UF1000i cut-off, bringing along an improvement in the screening parameters that would imply a high reduction in the workload and cost savings. In the community-dwelling elderly and according to our data, a 200bacteria/μL cut-off value should be used. This would mean reducing waiting times in the case of negative cultures, thus eliminating possible antibiotic adverse effects and interactions, with a minimal false negatives rate. Further prospective studies are needed in order to contrast these data with larger populations, and to establish the more convenient cut-off for each group of patients.
Conflict of interestThe authors declare no conflict of interest.