Chromoblastomycosis is a chronic infection, caused by pigmented fungi affecting skin and subcutaneous tissues characterized by verrucous nodules or plaques. Fonsecaea pedrosoi and Cladophialophora carrionii are the prevalent agents in the endemic areas. Phoma is an uncommon agent of human infection and involved mainly with phaeohyphomycosis cases. The case of a patient with a history of laceration in foot followed by verrucous aspect and scaly lesions, which had evolved for 27 years is presented. On physical examination disease was clinically compatible with chromoblastomycosis and the microscopic examination of scales showed fumagoid cells. On culture a dematiaceous fungus was grown. The agent was confirmed to be Phoma insulana based on its morphology and PCR-sequencing. This fungal agent has not been previously reported in association with this pathology.
La cromoblastomicosis es una infección crónica causada por hongos pigmentados que afecta la piel y el tejido subcutáneo y que se caracteriza por nódulos o placas verrugosas. Fonsecaea pedrosoi y Cladophialophora carrionii son los agentes prevalentes en las áreas endémicas. Phoma es un agente raro de infección humana y está involucrado principalmente en casos de feohifomicosis. Se presenta el caso de un paciente con antecedente de laceración en el pie, seguida de lesiones de aspecto verrugoso y descamativas, que evolucionaron durante 27años. En el examen físico la enfermedad fue clínicamente compatible con cromoblastomicosis y el examen microscópico de escamas mostró células fumagoides. En el cultivo creció un hongo dematiáceo. El agente fue confirmado como Phoma insulana en base a su morfología y PCR seguida de secuenciación. Este agente fúngico no ha sido reportado previamente en asociación con esta patología.
Chromoblastomycosis is an infection caused by the traumatic implantation of species of dematiaceous fungi, primarily in the skin and subcutaneous tissues of the lower limbs. The disease generally starts as a cutaneous nodule or papule which gradually increases in the adjacent areas and develops a scaly, greyish surface. After the lesion may evolve into one of the following described clinical forms: the nodular, tumoral, verrucous, cicatricial and plaque types. Histologically, a granulomatous reaction associated with acanthosis and pseudoepitheliomatous hyperplasia in the stratum corneum and epidermis is observed. The fungal structure in infected scales or tissues appears as rounded, dark-brown yeast-like bodies (5–15μm in diameter) with thick, planate-dividing walls that are known as sclerotic cells (also referred as “copper pennies,” “fumagoid cells,” “Medlar bodies,” or “muriform cells”).1
Chromoblastomycosis is a frequent disease in countries with tropical and subtropical climates, particularly in Latin America, Africa and Asia. The most frequent causative agents are Fonsecaea pedrosoi, F. compacta, Phialophora verrucosa, Cladophialophora carrionii, and Rhinocladiella aquaspersa. These fungi inhabit the soil and vegetal matter; therefore, the disease is more frequent in rural populations.2
This work describes the case of a patient living in deficient hygienic and socioeconomic conditions who presented a chronic subcutaneous infection compatible with chromoblastomycosis. The identified etiological agent has not been previously associated with this pathology.
CaseDuring a health campaign in rural areas organized by the Faculty of Chemical Sciences at the Autonomous University of Oaxaca the patient of this case was addressed. He was an indigent 79-year-old man, resident in Miahuatlan, Oaxaca (Mexico), and was a peasant with a history of chronic alcoholism and smoking. He began his current dermatological illness 27 years ago after the use of tight footwear that caused a laceration on his right heel. From that time, his lesion changed, with progressive thickening, pigmentation and peeling of the local skin. Several small nodules appeared and extended to the foot. The patient applied several empirical and aggressive treatments without improvement. He also sought medical assistance, but the treatment yielded unsatisfactory results. Five years ago, the presence of a progressive ulcerative lesion was detected.
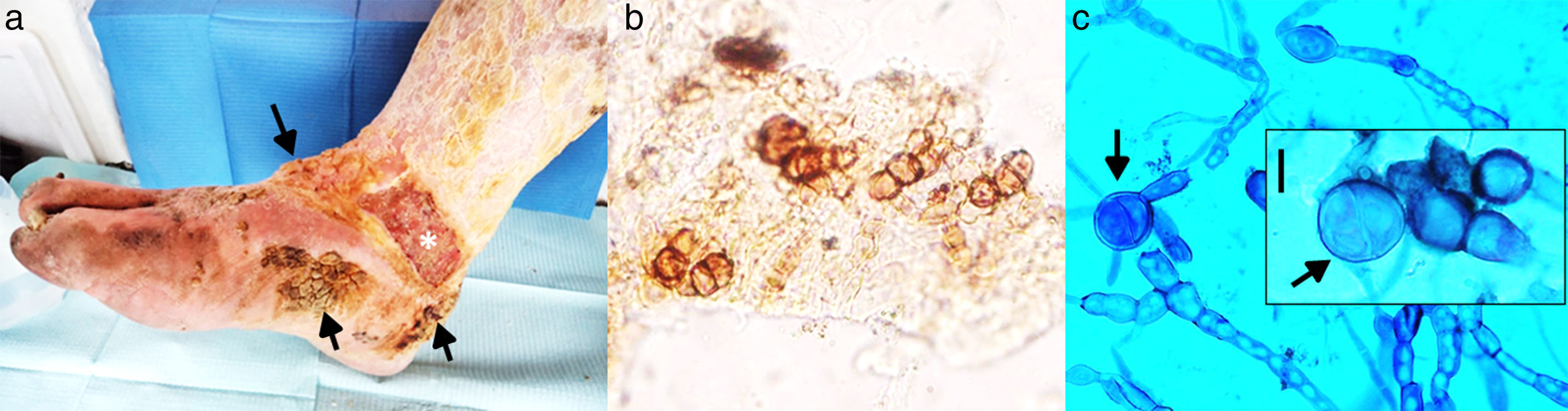
On physical examination, pigmented verrucous plaques were observed, predominantly on the anterior side, heel and internal side of the right foot. Moderate oedema and thick, yellow and adherent squamae were noteworthy on the leg. The patient had an ulcer located on the internal side of the lower third of the leg (10cm×5cm), with a well-defined border and a haematopurulent and foetid exudate. Additionally, larvae (miasis) were observed in the ulcer. The patient did not report symptoms localized in the affected zone but manifested asthenia, adynamia and general discomfort. He presented pallor, hearing loss, uncontrolled salivation, involuntary movements of both hands, and urinary incontinence. Authorization to take an image of his lesion was obtained (Fig. 1a). Additionally, scales and blood samples were collected for mycological study and analysis, respectively.
(a) Pigmented verrucous lesions on the foot (arrows), an ulcerative lesion (*) on the lower part of the leg, and yellowish, thick, crusty lesions on the leg are observed. (b) Microscopic examination of scales, showing thick walled, round-to-ovoid brown cells with septa characteristic of fumagoid cells (40×). (c) Microscopic examination with lactophenol blue from primary culture: irregular and pigmented hyphae with internal guttules, chlamydoconidia-like cells and some septate globose structures similar to fumagoid cells (arrows) are observed (scale bar: 10μm).
Haematological analysis revealed values within normal parameters.
Mycological studyMicroscopic examination of the skin scales with 20% potassium hydroxide showed individual or grouped, brown, thick-walled, planate-dividing round cells, 10μm×13μm in diameter, compatible with fumagoid cells (Fig. 1b). The scales were cultivated on Sabouraud dextrose agar (SDA) with and without antibiotics at 28°C, with periodic revision. After two weeks, several small, pigmented, downy colonies were observed on SDA without antibiotics. No growth was present on SDA with antibiotics, and these tubes were discarded after three weeks of incubation. Microscopic examination of the primary culture revealed pigmented and irregular hyphae, and swollen cells, insufficient features to identify the fungus (Fig. 1c).
After observation of fumagoid cells in scales and a dematiaceous fungus in culture, the chromoblastomycosis diagnosis was established. The patient was visited at home and informed about his disease. However, he refused any topical or systemic treatment, and a follow-up visit was not possible. After the final identification of the fungal isolate, a colleague again went to visit the patient around three months later. The neighbours informed about the patient's death due to “cardiac failure”.
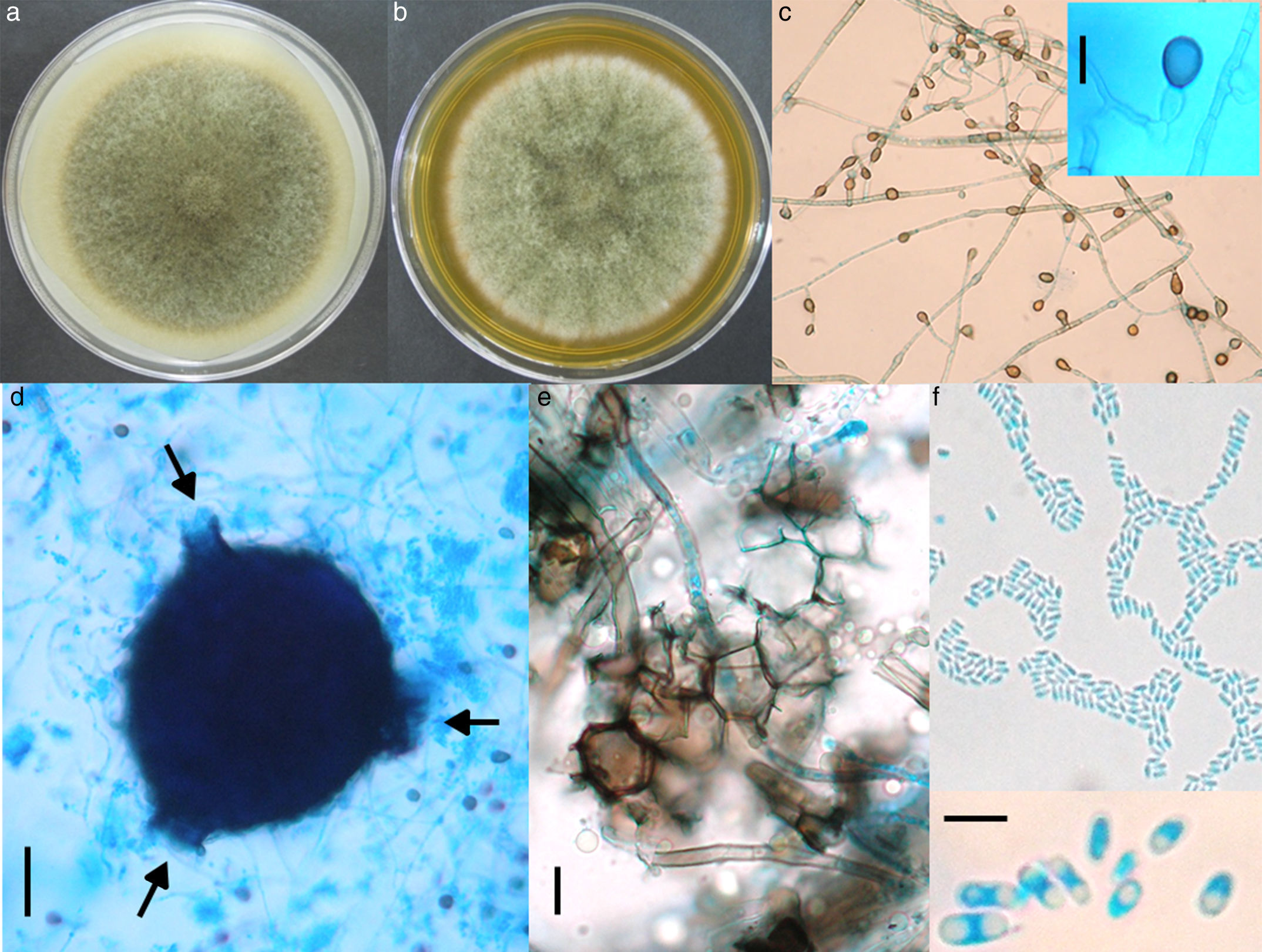
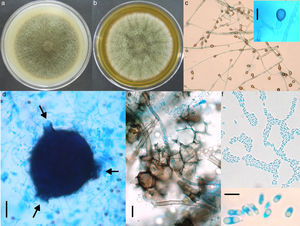
Morphological studyThe fungus was grown on SDA, on potato dextrose agar (PDA) and on lactrimel agar (LA) for 6 days at 28°C. The microscopic morphology on SDA and PDA was similar to that observed for the primary isolate, but it revealed numerous chlamydoconidia on LA. After four weeks on PDA, many irregular and round pycnidia were observed. The fungus was later grown on malt extract agar (MEA) and on oat agar (OA) for 8 days at 28°C (Fig. 2a and b). The size of colony was 7.0 and 6.7cm of diameter, respectively. Microscopic morphology on two media was similar, but only description on OA is done. Abundant globose or piriformis, intercalary, terminal or in chains, of variable size (5–14μm diameter) chlamydospores, were observed (Fig. 2c). Numerous globose (200μm), piriformis (200μm×360μm) or irregular pycnidia, with one to three ostioles or pores (Fig. 2d) were observed. Polyhedral cells formed the pycnidial wall (Fig. 2e). Abundant ellipsoidal, hyaline conidia with one or two polar guttules emerging from picnidia were present (Fig. 2f). Conidial matrix whitish was evident. The morphological characteristics were integrated according to Boerema et al.3
Culture on OA (a) and MEA (b) after 8 days of growth, showing pigmented, woolly, green olivaceous colonies. (c) Abundant pigmented chlamydoconidia (10×); insert: magnification of a chlamydoconidium (scale bar: 10μm). (d) Globose picnidium (scale bar: 50μm), showing three ostiolae (arrows). (e) Polymorphic cells of the picnidium wall (scale bar: 10μm). (f) Abundant small and ellipsoidal conidia (40×); insert: magnification of conidia (scale bar: 5μm).
DNA was extracted from a monosporic culture in Sabouraud dextrose broth using the GeneAll Exgene Plant SV mini kit (GeneAll Biotechnology Co., Ltd., Seoul, Korea). Three PCR reactions were performed to target the ITS region, the actin gene and the beta-tubulin gene, according to the methodology of the Q-bank Fungi database for Phoma and Phoma-like fungi. The amplified fragments were purified using the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) and sequenced in two directions using an ABI3130/3130xl Genetic Analyzer (Applied Biosystems/Hitachi, Foster City, CA, USA). The sequences were analyzed according to the Q-bank Fungi database. The results of this analysis indicated a high similarity with Phoma insulana CBS 252.92 (ITS and ACT 100%, GenBank GU237810; TUB 99.676%, GenBank GU237618). The molecular and morphological studies were compatible. The nucleotide sequences were deposited in GenBank under the accession numbers KR921746 (ITS), KT163805 (ACT), and KT163806 (TUB). The strain was deposited in the Colección de Microorganismos, Centro de Investigación y Estudios Avanzados, Instituto Politécnico Nacional, México, as CDBB-H-1229.
Antifungal susceptibility testingThe microdilution broth testing was performed in duplicate for itraconazole, voriconazole, posaconazole, fluconazole and amphotericin B, following the recommendations of the Clinical Laboratory Standard Institute (CLSI) M-38 A2 for filamentous fungi.4 The MICs for P. insulana were: itraconazole and posaconazole, 0.06μg/mL; voriconazole 0.25μg/mL; fluconazole 16.0μg/mL, and amphotericin B 2.0μg/mL. Therefore the highest antifungal activity corresponded to itraconazole and posaconazole.
DiscussionPhoma (Saccardo 1880; Sacc emend. Boerema & G.J. Bollen) is an anamorphic, complex constituted by dematiaceous fungi. These organisms inhabit soil, organic debris, and water and include species that parasitize other fungi, insects and vertebrates. Approximately 10 species are reported as opportunistic or primary human pathogens.5
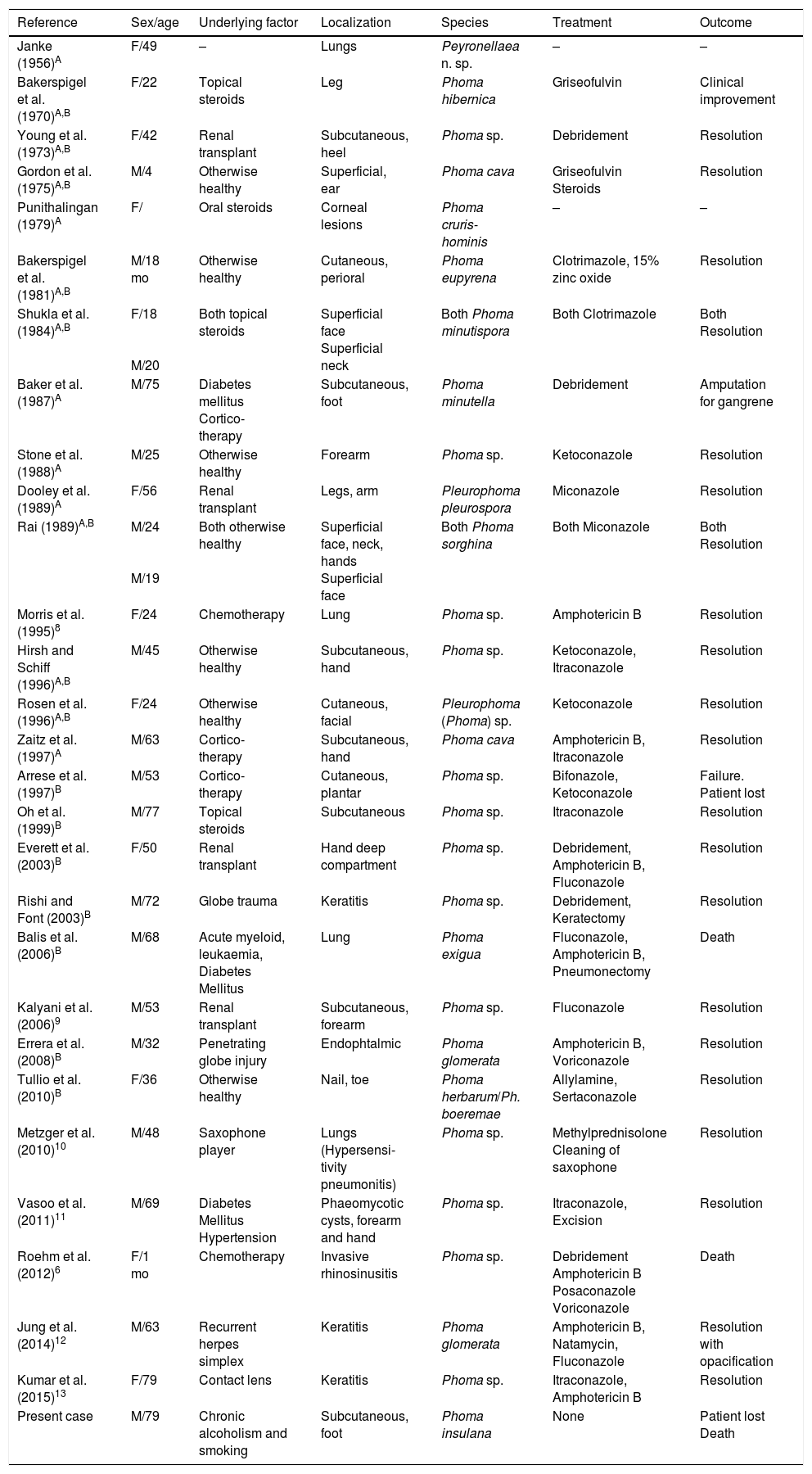
In 1970, Bakerspigel (cited in 6 and 7) reported the first case of human infection caused by Phoma (Phoma hibernica). Currently there are at least 30 reports of human infections caused by Phoma spp. as shown in Table 1.6–13
Literature review of human pathology cases associated with Phoma spp.
| Reference | Sex/age | Underlying factor | Localization | Species | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Janke (1956)A | F/49 | – | Lungs | Peyronellaea n. sp. | – | – |
| Bakerspigel et al. (1970)A,B | F/22 | Topical steroids | Leg | Phoma hibernica | Griseofulvin | Clinical improvement |
| Young et al. (1973)A,B | F/42 | Renal transplant | Subcutaneous, heel | Phoma sp. | Debridement | Resolution |
| Gordon et al. (1975)A,B | M/4 | Otherwise healthy | Superficial, ear | Phoma cava | Griseofulvin Steroids | Resolution |
| Punithalingan (1979)A | F/ | Oral steroids | Corneal lesions | Phoma cruris-hominis | – | – |
| Bakerspigel et al. (1981)A,B | M/18 mo | Otherwise healthy | Cutaneous, perioral | Phoma eupyrena | Clotrimazole, 15% zinc oxide | Resolution |
| Shukla et al. (1984)A,B | F/18 M/20 | Both topical steroids | Superficial face Superficial neck | Both Phoma minutispora | Both Clotrimazole | Both Resolution |
| Baker et al. (1987)A | M/75 | Diabetes mellitus Cortico-therapy | Subcutaneous, foot | Phoma minutella | Debridement | Amputation for gangrene |
| Stone et al. (1988)A | M/25 | Otherwise healthy | Forearm | Phoma sp. | Ketoconazole | Resolution |
| Dooley et al. (1989)A | F/56 | Renal transplant | Legs, arm | Pleurophoma pleurospora | Miconazole | Resolution |
| Rai (1989)A,B | M/24 M/19 | Both otherwise healthy | Superficial face, neck, hands Superficial face | Both Phoma sorghina | Both Miconazole | Both Resolution |
| Morris et al. (1995)8 | F/24 | Chemotherapy | Lung | Phoma sp. | Amphotericin B | Resolution |
| Hirsh and Schiff (1996)A,B | M/45 | Otherwise healthy | Subcutaneous, hand | Phoma sp. | Ketoconazole, Itraconazole | Resolution |
| Rosen et al. (1996)A,B | F/24 | Otherwise healthy | Cutaneous, facial | Pleurophoma (Phoma) sp. | Ketoconazole | Resolution |
| Zaitz et al. (1997)A | M/63 | Cortico-therapy | Subcutaneous, hand | Phoma cava | Amphotericin B, Itraconazole | Resolution |
| Arrese et al. (1997)B | M/53 | Cortico-therapy | Cutaneous, plantar | Phoma sp. | Bifonazole, Ketoconazole | Failure. Patient lost |
| Oh et al. (1999)B | M/77 | Topical steroids | Subcutaneous | Phoma sp. | Itraconazole | Resolution |
| Everett et al. (2003)B | F/50 | Renal transplant | Hand deep compartment | Phoma sp. | Debridement, Amphotericin B, Fluconazole | Resolution |
| Rishi and Font (2003)B | M/72 | Globe trauma | Keratitis | Phoma sp. | Debridement, Keratectomy | Resolution |
| Balis et al. (2006)B | M/68 | Acute myeloid, leukaemia, Diabetes Mellitus | Lung | Phoma exigua | Fluconazole, Amphotericin B, Pneumonectomy | Death |
| Kalyani et al. (2006)9 | M/53 | Renal transplant | Subcutaneous, forearm | Phoma sp. | Fluconazole | Resolution |
| Errera et al. (2008)B | M/32 | Penetrating globe injury | Endophtalmic | Phoma glomerata | Amphotericin B, Voriconazole | Resolution |
| Tullio et al. (2010)B | F/36 | Otherwise healthy | Nail, toe | Phoma herbarum/Ph. boeremae | Allylamine, Sertaconazole | Resolution |
| Metzger et al. (2010)10 | M/48 | Saxophone player | Lungs (Hypersensi-tivity pneumonitis) | Phoma sp. | Methylprednisolone Cleaning of saxophone | Resolution |
| Vasoo et al. (2011)11 | M/69 | Diabetes Mellitus Hypertension | Phaeomycotic cysts, forearm and hand | Phoma sp. | Itraconazole, Excision | Resolution |
| Roehm et al. (2012)6 | F/1 mo | Chemotherapy | Invasive rhinosinusitis | Phoma sp. | Debridement Amphotericin B Posaconazole Voriconazole | Death |
| Jung et al. (2014)12 | M/63 | Recurrent herpes simplex | Keratitis | Phoma glomerata | Amphotericin B, Natamycin, Fluconazole | Resolution with opacification |
| Kumar et al. (2015)13 | F/79 | Contact lens | Keratitis | Phoma sp. | Itraconazole, Amphotericin B | Resolution |
| Present case | M/79 | Chronic alcoholism and smoking | Subcutaneous, foot | Phoma insulana | None | Patient lost Death |
ACited in 6; BCited in 7. Species names are given as reported by authors. The following current taxonomic names were taken from Index Fungorum. Peyronellaea: included in Phoma section Peyronellaea; Phoma cava: Pyrenochaeta cava; Phoma minutispora: Westerdikella minutispora; Pleurophoma pleurospora: Dinemasporium pleurospora; Phoma sorghina: Epicoccum sorghinum; Phoma exigua: Boeremia exigua.
Different therapeutic resources have been used in clinical cases, most with satisfactory outcomes. In a recent in vitro antifungal susceptibility study, posaconazole and voriconazole showed the best activity against dematiaceous fungi including Phoma isolates. In that study amphotericin B and fluconazole were the least active drugs.14 These last findings are similar to those observed for P. insulana.
Five genera of dematiaceous fungi are the main causative agents of chromoblastomycosis in the world. In Mexico the frequency of the agents is: F. pedrosoi (95.8%), C. carrionii (1.1%), P. verrucosa (0.6%), R. aquaspersa (0.2%) and E. spinifera (0.2%).15 Here we inform an additional genus associated with this pathology.
In this work we report a case diagnosed as chromoblastomycosis based on the following data: (a) Disease began after skin trauma. (b) The clinical manifestations consisted of lesions with verrucous aspect and chronic evolution. (c) The microscopic examination of scales showed fumagoid cells, fungal structures associated only to chromoblastomycosis. (d) On culture developed several colonies of a dematiaceous fungus, kind of fungi described as etiological agents of phaeohyphomycosis or chromoblastomycosis. Due to patient’ reluctance, it was not possible to carry out the histopatological study which would reveal in addition fumagoid cells, a tissue response consisted of pseudoepitheliomatous hyperplasia, acanthosis and hyperkeratosis. Accordingly it was not possible prescribe him an appropriate treatment.
To the best of our knowledge, this is the first report of chromoblastomycosis caused by P. insulana.
Conflict of interestThe authors declare no conflict of interest.
This study was supported by the Faculty of Medicine, Universidad Nacional Autonoma de Mexico (UNAM). We thank VK Espinoza-Sánchez for contributing cultures for Phoma insulana maintenance.