Microbiological Diagnostic Stewardship (MIDS) Programs promote coordinated measures aimed at optimizing the use of diagnostic techniques, thus favouring the adoption of adequate and cost-effective therapeutic, clinical and preventive decisions. The implementation of MIDS relies upon the creation of multidisciplinary committees led by clinical microbiologists for the design of diagnostic algorithms, the adequacy of the laboratory computer system (LCS) to monitor the relevance of the requested diagnostic tests, the implementation of a quality control system, the design and performance of studies of cost-effectiveness, the training of the petitioner and the technical and nursing staff and the continuous evaluation of the program. The incorporation of MIDS in routine care reports tangible benefits for the patient while strengthening the pivotal role of the clinical microbiologist in the management of infectious diseases.
Los Programas de Optimización del Diagnóstico Microbiológico (PRODIM) promueven actuaciones coordinadas orientadas a optimizar el uso de las técnicas diagnósticas, propiciando la toma de decisiones terapéuticas, clínicas y preventivas adecuadas y “coste-efectivas”. La implementación de los PRODIM debe sustentarse en la creación de comités multidisciplinares liderados por microbiólogos clínicos para el diseño de algoritmos diagnósticos, la adecuación del sistema informático del laboratorio (SIL) para el control de la pertinencia de las pruebas diagnósticas solicitadas, la puesta en marcha de un sistema de control de calidad, el diseño y realización de estudios de análisis de coste-efectividad, la educación del peticionario y del personal técnico y de enfermería y la evaluación continua del programa. La incorporación de PRODIM en la rutina asistencial reporta beneficios tangibles para el paciente a la vez que afianza el papel clave del microbiólogo clínico en el manejo de las enfermedades infecciosas.
The concept of the Programa de Optimización del Diagnóstico Microbiológico [Microbiological Diagnostic Optimisation Programme] (PRODIM) is linked to the concept of diagnostic stewardship. According to the Global Antimicrobial Resistance Surveillance System manual, endorsed by the World Health Organization,1 diagnostic stewardship is "coordinated guidance and interventions to improve appropriate use of microbiological diagnostics to guide therapeutic decisions". PRODIMs push the conceptual boundaries of diagnostic stewardship in that they incorporate the notion that the implementation of a diagnostic test in the catalogue of services provided by a microbiology laboratory must be supported by a favourable cost-opportunity analysis, and that the diagnostic test not only must prove clinically useful, but also should be "cost-effective".2–10 In this sense, PRODIMs are aligned with the postulates of the "Choosing Wisely" campaign (http://www.choosingwisely.org/), which are endorsed by two study groups belonging to the European Society of Clinical Microbiology and Infectious Diseases, the Study Group for Genomic and Molecular Diagnostics and the Study Group for Antimicrobial Stewardship,11 and advocate that diagnostic tests must be carefully selected to ensure "improvement in health" while being cost-effective for the system that funds them. In this context, Royal Decree (RD) 16/2012 of 20 April points to a need for training in economic evaluation studies for health technologies, including diagnostic tests, in line with RD 9/2011 of 19 August, which already indicated that the cost-effectiveness criterion had to be an official requirement to negotiate the price and funding of medicines.12,13
Although PRODIMs, like diagnostic stewardship programmes, were created in connection with antimicrobial use optimisation programmes,14 their application in the management of infectious diseases currently exceeds the mere purpose of suitably guiding the selection of antimicrobials, since they pursue clinical and preventive decisions of any nature that improve patient and community health at a cost that can be assumed by the health system.15
Why PRODIMs?Clinical microbiology laboratories are at the epicentre of a global diagnostic revolution brought about by the advent of new molecular diagnostic technologies based on amplification, hybridisation or sequencing of microbial nucleic acids or coupled with the use of magnetic resonance imaging and proteomics (mass spectrometry — MALDI-TOF),16 the implementation of which in most Spanish hospitals has not met with major obstacles. The use of this technology enables a diagnosis to be made in less time compared to a few years ago, when traditional culture and rapid methods based on detection of microbial antigens and specific antibodies (serology) were dominant. However, the incorporation of these technological advances entails practical challenges not only from a logistical point of view for laboratories, but also for ordering clinicians: "our technical capacities are exceeding our capacity to apply them effectively and reasonably in solving clinical problems".17,18 Microbiology laboratories today face problems of different natures and magnitudes, the enumeration and analysis of which fall outside of the scope of this review. In our opinion, they are especially relevant in relation to PRODIMs. On the one hand, there is a great deal of pressure on the healthcare system to perform "syndromic" tests capable of simultaneously detecting a wide variety of microorganisms that may be involved in the aetiology of a certain disease; these are often expensive and lack reasonable clinical support. On the other hand, there is a dilemma between providing or not providing information whose clinical significance may be questionable.
The implementation of PRODIMs minimises the risk of "overdiagnosis" while promoting suitable use of antimicrobials, resulting in a substantial improvement in patient safety and care.11–15 However, PRODIMs are not exempt from "undesired effects", since improving the pretest positive predictive value of the tests can lead to "overlooking" some diagnoses and limit the clinician's autonomy in ordering diagnostic tests. This is a potential source of tension that can inevitably be detrimental to good medical care.19
Fundamentals of PRODIMsThe process of diagnosis is based on the so-called "brain-to-brain loop" concept developed by Lundberg.20,21 It begins with the formulation of a series of linked questions: does the patient have an infection? If so, what is the causative microorganism? How should I treat it?17,22 It ends when test results are interpreted and lead to a resulting clinical action (actionable results). This clinical microbiologists ensure the suitability of pretesting processes (sample/patient reconciliation and correct sample selection, collection, transport and processing), testing processes (test execution in compliance with recognised quality standards) and post-testing processes (issuance of intelligible, judicious, clinically significant reports as quickly as possible). Ultimately, the effectiveness and efficiency of the process of diagnosis depend on productive collaboration among all parties involved: clinical microbiologists, ordering clinicians, laboratory technicians, auxiliary and nursing personnel, and, of course, managers. The end result of this collaboration should be captured in microbiological diagnostic guidelines adapted to the reality and the possibilities of each health department.
Selection of the right diagnostic testUnsuitable choices of microbiological diagnostic tests, due to either overuse (tests are ordered, but are not indicated) or underuse (tests are indicated, but are not ordered), presumably represent a global phenomenon,23 although the magnitude thereof can be predicted to vary in relation to the degree of restrictiveness or permissiveness of the criteria in use that govern the indication for a test at a given centre. The direct cost of unsuitable orders for microbiology laboratories is probably negligible, considering that the diagnostic tests performed in the laboratories (as a whole, not just in microbiology laboratories) represent a small fraction (3%–5%) of all health expenditure.24 However, so-called costs that can be attributed to decisions deriving from test results should be added to direct costs and include drug prescriptions, imaging tests, surgical procedures and hospital admissions.
This is not a question of defining responsibilities or assigning them to the different participants in the diagnostic process, because in one way or another, everybody — microbiologists, clinicians, managers and even patients — is responsible to a greater or lesser extent. Recently, Salina et al.25 correctly indicated some of the causes of the huge numbers of orders for inappropriate laboratory tests (the researchers were referring to general clinical analysis laboratories, but their findings can be extrapolated to specialised microbiology laboratories). With regard to clinical microbiologists, the incorporation into the service portfolios of techniques whose efficacy and effectiveness have not been firmly established; the excess of "order profiles", fed by electronic order systems; and the implementation in the laboratory of diagnostic algorithms with reflex tests (not ordered by the ordering clinician), the clinical value of which has not been sufficiently verified, are factors that reduce the suitability of the orders (without claiming to offer a comprehensive list of them).
To summarise, PRODIM policies are aimed at choosing the right diagnostic test for a specific patient at the optimal time, in order to act with maximum clinical efficacy and "cost-effectiveness" (the "right test for the right patient at the right time with the right cost for producing the right outcome"). But how is the relevance of a diagnostic test rated? The robustness of the available scientific evidence must undoubtedly be the primary criterion on which the choice of one test or another is based. In this context, the European Parliament recently approved the new European Regulation 2017/74640, which governs and regulates the marketing authorisation of in vitro diagnostic tests in the countries of the European Union.26 According to this regulation, obtaining CE marking requires accreditation that said test is scientifically valid — in other words, that it is technically suitable in terms of diagnostic need that it is intended to meet, documentation of its intrinsic testing characteristics (veracity-bias, precision-repeatability and reproducibility-accuracy, limits of detection and quantification, measurement range, and linearity), specification of pretesting requirements (sample collection and handling and interference with measurement by exogenous and endogenous substances) for its execution. Obtaining CE marking also requires listing the clinical performance of the in terms of diagnostic sensitivity and specificity and positive and negative predictive values, taking into account the prevalence of the disease in question, the verisimilitude ratio and the expected values in healthy and diseased populations. This legislation also requires that the validation be performed through a clinical trial that mimics the situation of diagnostic need for which it has been designed ("in real life").
However, the scientific literature often raises more doubts than certainties. Hence, in the absence of conclusive data, a decision to prioritise one test over another must be agreed upon between ordering clinicians and microbiologists; must be as closely aligned as possible with expert recommendations, if any; and must be explicitly indicated in clinical action documents or guidelines adapted to a local reality, since the clinical utility and cost-effectiveness of a specific diagnostic test can vary considerably, depending on the type of patient on whom it is performed.
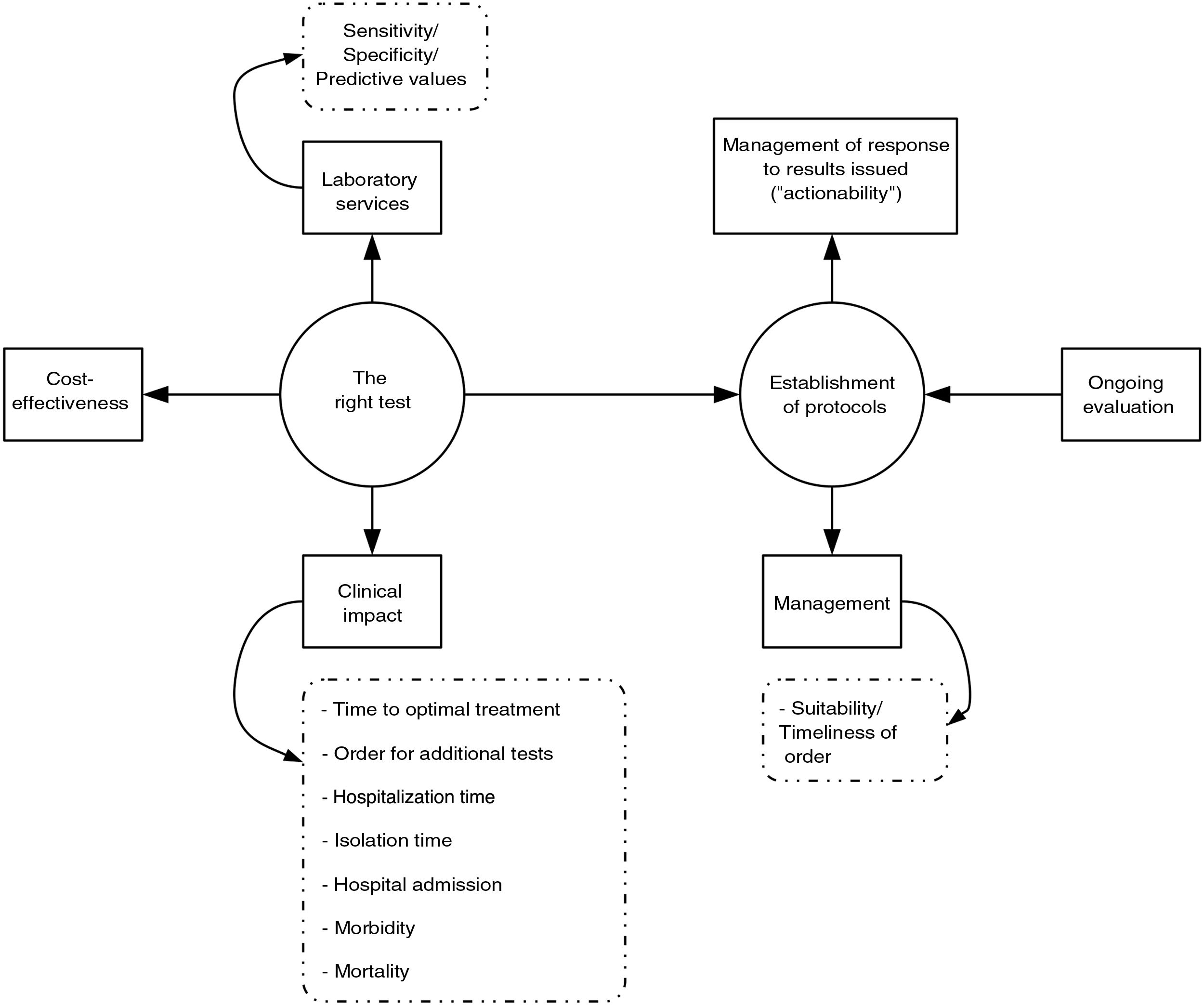
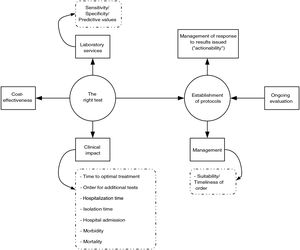
Implementation of PRODIMsIn our opinion, the implementation of PRODIMs should be based on the following actions (Fig. 1): (a) design of a portfolio of services that incorporates tests of proven clinical value; avoids duplication; and adapts, to the extent possible, to the economic and financial possibilities of the corresponding hospital. It should include response times. (b) Creation of multidisciplinary committees (with other departments), to be led by clinical microbiologists, to design diagnostic algorithms, sample acceptance/rejection policy, reflex test routes; make decisions on "relocation" of diagnostic tests (point of care); determine desirable response times; prepare report contents; and schedule "actionables", i.e., pre-established medical actions in response to microbiological test results (especially rapid test results). Undoubtedly, close collaboration between microbiology laboratories and medical or surgical departments results in better patient care, which minimises the morbidity and mortality associated with the infectious disease in question. Clinical management of bacteraemia is a paradigmatic example that proves this claim. The clinical impact of rapid diagnosis using MALDI-TOF or molecular genotyping tests is indeed substantially higher when an antibiotic use optimisation programme team is involved in adjusting empirical treatment.27–32 (c) Laboratory information systems (LIS) suited to managing the relevance of the diagnostic tests ordered that enable restriction of access to said tests according to the origin of an order and when an order is not duly justified. The use of programs aimed at this purpose has been shown to be useful in managing drug prescription suitability (computerised provider order entry and clinical decision support systems).33 (d) Implementation of a system for quality control (sample processing, testing and post-testing) in diagnostic tests. (e) Design and conduct of studies to analyse the cost-effectiveness of the diagnostic tests or algorithms to be implemented. (f) Education of ordering clinicians (update on the diagnostic technology used, services and interpretation of the results of the diagnostic tests; etc.) and education of the technical and nursing staff (collection and processing of samples, execution of diagnostic tests, etc.). (g) Continuous evaluation of the programme (regular reports, benchmarking, feedback for ordering clinicians, etc.).
Microbiology laboratories must promote technological innovation and adoption of diagnostic tests with PRODIM criteria as a priority action. The incorporation of PRODIMs into routine healthcare brings tangible benefits for patients while strengthening the key role of clinical microbiologists in managing infectious diseases.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to thank MSD for their help with organising the logistics of the PRODIM 1 and PRODIM 2 meetings, held in Madrid in June 2018 and 2019, respectively, during which some of the ideas in this manuscript were outlined.
Please cite this article as: Bou G, Cantón R, Martínez-Martínez L, Navarro D, Vila J. Fundamentos e implementación de Programas de Optimización de Diagnóstico Microbiológico. Enferm Infecc Microbiol Clin. 2021;39:248–251.