A 56-year-old previously healthy Mexican woman presented to evaluation with a three-month history of diarrhea (up to 20 stools per day, with mucus and without blood), weight loss, and anasarca. She lived in a Mexican rural area with a tropical climate (Minatitlán, Veracruz), and was engaged in household chores, the past medical history she denied chronic diseases, alcohol habit, tobacco, drug or steroid therapy use or as well as trips in the last year.
At the physical exam, temperature was 37.0°C, the blood pressure was 90/60mmHg, pulse 90 beats per minute, respirations 18 breaths per minute and oxygen saturation of 96% on room air. The patient appeared in discomfort. The abdomen was distended, with generalized tenderness, increased peristaltic movements, and ascitis.
Laboratory results showed a normal WBC count (5.4×109/L with 2.5% of eosinophils), normocytic normochromic anemia (Hb 10.3g/dL), and evidence of malabsorption (albumin 2.4g/dL, folic acid 3.22ng/mL, serum carotene concentration 19mcg/dL) the serum creatinine was 0.34mg/dL (reference range 0.6–1.2mg/dL), serum sodium was 134mmol/L (reference range 135–145mmol/L), serum potassium was 2.21mmol/L (reference range 3.5–4.5mmol/L), clinical urine test was normal with out proteinuria, total Bilirrubin 1.31 (reference range 1–1.2mg/dL) ALT 11.3 AST 27 (reference ranges for both 8–40U/L). Arterial blood gas analysis reported pH 7.55 (7.35–7.45), paCO2 34.4mmHg (reference range 35–45mmHg), HCO3 30.1mmol/L (reference range 22.2–28mmol/L).
Diagnosis and evolutionFurther diagnostic workup included serum anti-gliadin antibodies, fecal elastase test, fourth-generation HIV testing, anti liver/kidney microsome (LKM 1), antimitochondrial Ab, and stool ova and parasites analysis, all with negative results, the HTLVI antibody screen was not carried out, paracentesis showed cell count 25PMNs/mm3, total protein 2.69g, serum ascites albumin gradient (SAAG) 1.4, serologic test for S stercolaris was positive, CT imaging revealed diffuse colonic and small bowel wall thickening, ascites, and pleural effusion (Fig. 1A and B). An upper and lower endoscopy was performed and revealed colonic mucosa erythema and edema. Duodenal and colonic biopsies showed granulomas with eosinophils and larvae compatible with Strongyloides stercoralis (Fig. 1C–G), Ivermectin (200mcg/kg/day×2 days) was administered, for two cycles, and after a few months, she had a complete recovery.
Final commentStrongyloides stercoralis is an intestinal nematode that infects humans when their skin is exposed to soil contaminated with infective larvae. Between 30 and 100 million people are infected worldwide. Strongyloidiasis is seen predominantly in resident populations from endemic areas in the tropics and subtropics.1 Although it is known to be a cause of chronic diarrhea, the burden of the disease has been felt to be underestimated.2
In the immunocompetent host, strongyloidiasis usually causes mild gastrointestinal symptoms or asymptomatic eosinophilia. Notwithstanding, given that S. stercoralis has the capability of completing its life cycle within humans, it can produce other syndromes such as chronic diarrhea and wasting or, mainly in immunocompromised patients, the hyperinfection syndrome.1
Gastrointestinal symptoms are common but are non-specific; Abdominal pain, watery diarrhea, constipation, anorexia, weight loss, difficulty swallowing, nausea, vomiting, gastrointestinal bleeding, and small bowel obstruction may occur.2 Protein-losing enteropathy may give rise to acute or worsening hypoalbuminaemia with peripheral oedema or ascites.2 Hypokalaemia or other electrolyte abnormalities may reflect these gastrointestinal disturbances, as presented in this clinical case.
In our case, the presence of hyperinfection syndrome was ruled out, the patient was theoretically immunocompetent, without the use of immunosuppressive drugs, remember that the main risk factor for presenting hyperinfection is secondary cellular immunosuppression, or a history of chronic debilitating diseases.
The symptoms of hyperinfection syndrome are produced by the direct invasion of the parasite associated with secondary bacterial infections, it generally presents with fever, dyspnea, vomiting, abdominal pain, forms of severe pneumonia, septic shock, meningitis, in our case it did not manifest with severe forms in addition to the fact that the mortality of this clinical syndrome is high between 30 and 80% and our patient recovered and did not die.
Diagnosis and effective therapy are essential in order to eradicate the infection and the life-long risk involved.
In chronic infections, the diagnostic sensitivity of stool parasite determination techniques is generally low, although it could increase with the repetition of several samples. The serology is usually positive and remains in time even after treatment, in addition to presenting cross-reactions with other helminths, or false negatives in patients with compromised cellular and humoral immunity. In hyperinfection syndrome, visualization is more likely due to the high number of parasites in feces, in bronchoalveolar lavage, gastroduodenal aspirate, sputum or in body fluids, such as pleural fluid, ascites, cerebrospinal fluid and even urine test.3
The most histopathologic alterations of gastric o duodenal mucosa associated with the presence of strongiloides parasites reveal stercoralis larvae, may eggs, some adult forms, in the gastric crypts, duodenal glands, or eosinophilic infiltration in the lamina propria.4
Ivermectin is the drug of choice against S. stercoralis; the therapeutic efficacy remains above 90% and is more effective than a 7-day course of high dose albendazole for patients with infection due to S. stercoralis.5,6
In conclusion, in patients with a relevant epidemiological background, stool ova and parasite analysis should be considered in the workup of patients with chronic diarrhea and wasting. In the case of negative results, an endoscopy could provide further diagnostic information.






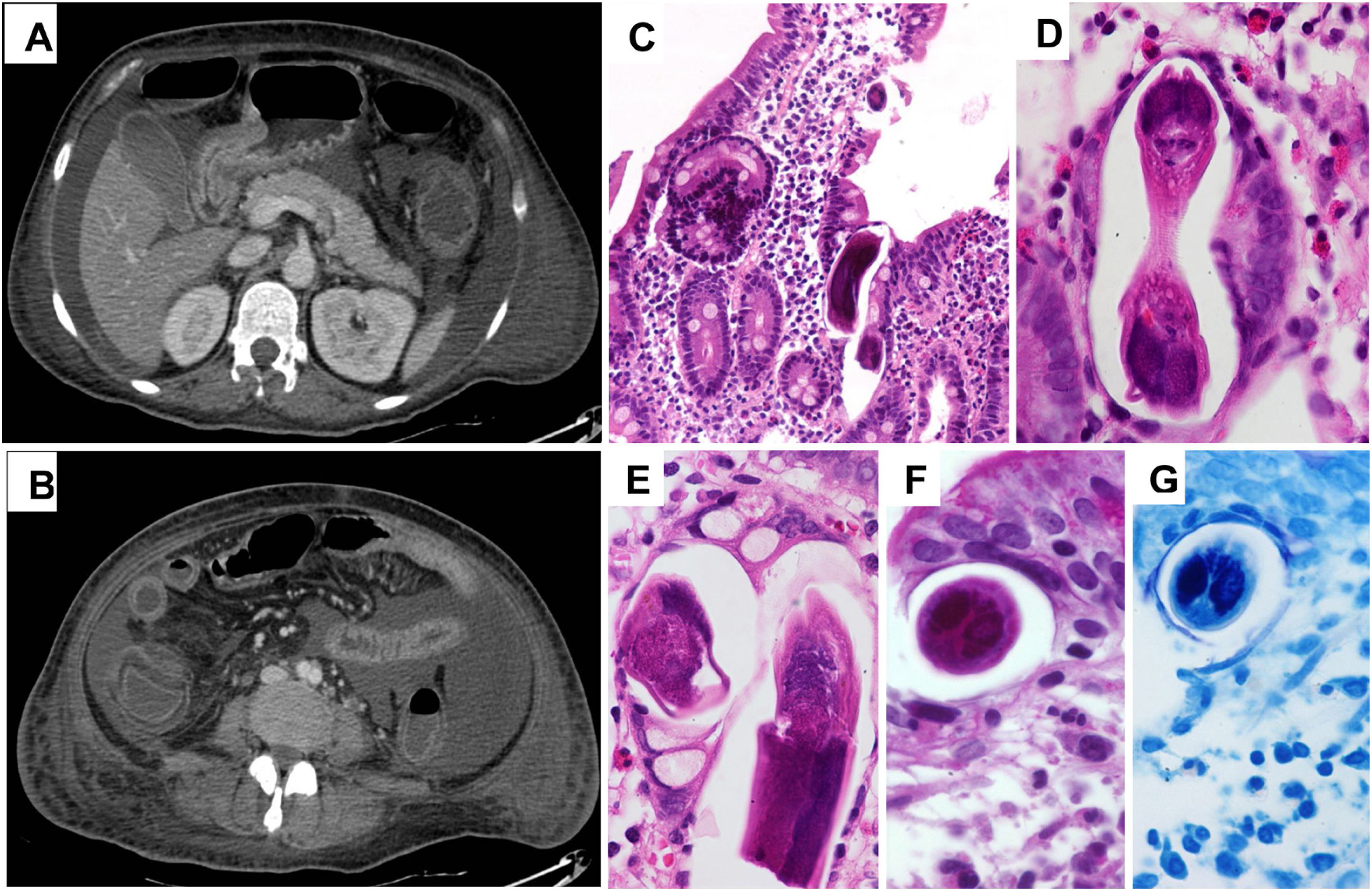
![(A, B) Abdominal CT scan showing ascites in addition to small and large bowel wall thickening. (C–G) Duodenal biopsies showing S. stercoralis larvae and abundant eosinophils in the lamina propria (H & E [C–F] and PAS [G] stainings). (A, B) Abdominal CT scan showing ascites in addition to small and large bowel wall thickening. (C–G) Duodenal biopsies showing S. stercoralis larvae and abundant eosinophils in the lamina propria (H & E [C–F] and PAS [G] stainings).](https://static.elsevier.es/multimedia/2529993X/0000003900000005/v1_202105020729/S2529993X21000897/v1_202105020729/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
