The COVID-19 pandemic has affected the care of patients with other diseases. Difficulty in access to healthcare during these months has been especially relevant for persons with HIV infection (PWH). This study therefore sought to ascertain the clinical outcomes and effectiveness of the measures implemented among PWH in a region with one of the highest incidence rates in Europe.
MethodsRetrospective, observational, pre-post intervention study to compare the outcomes of PWH attended at a high-complexity healthcare hospital from March to October 2020 and during the same months across the period 2016–2019. The intervention consisted of home drug deliveries and preferential use of non face-to-face consultations. The effectiveness of the measures implemented was determined by reference to the number of emergency visits, hospitalisations, mortality rate, and percentage of PWH with viral load >50copies, before and after the two pandemic waves.
ResultsA total of 2760 PWH were attended from January 2016 to October 2020. During the pandemic, there was a monthly mean of 106.87 telephone consultations and 2075 home deliveries of medical drugs dispensed to ambulatory patients. No statistically significant differences were found between the rate of admission of patients with COVID-HIV co-infection and that of the remaining patients (1172.76 admissions/100,000 population vs. 1424.29, p=0.401) or in mortality (11.54% vs. 12.96%, p=0.939). The percentage of PWH with viral load >50copies was similar before and after the pandemic (1.20% pre-pandemic vs. 0.51% in 2020, p=0.078).
ConclusionOur results show that the strategies implemented during the first 8 months of the pandemic prevented any deterioration in the control and follow-up parameters routinely used on PWH. Furthermore, they contribute to the debate about how telemedicine and telepharmacy can fit into future healthcare models.
La pandemia causada por el SARS-CoV-2 ha afectado a la atención de pacientes con otras enfermedades. La dificultad en el acceso a la asistencia sanitaria durante estos meses es especialmente relevante en las personas con infección por VIH (PCV). El objetivo del estudio fue conocer los resultados clínicos y la efectividad de las medidas implementadas en PCV en una de las regiones con mayor incidencia de Europa.
MétodosEstudio observacional retrospectivo, pre-postintervención, comparando los resultados de PCV atendidos en un hospital de alta complejidad entre marzo-octubre de 2020 y el mismo periodo de 2016 a 2019. La intervención consistió en el envío a domicilio de medicamentos y la realización preferente de consultas no presenciales. La efectividad de las medidas implementadas se determinó por el número de visitas a urgencias, hospitalizaciones, mortalidad y porcentaje de PCV con carga viral>50 copias antes y después de 2 olas pandémicas.
ResultadosSe atendieron 2.760 PCV entre enero de 2016 y octubre de 2020. Durante la pandemia se realizaron una media mensual de 106,87 consultas telefónicas y 2.075 envíos a domicilio de medicamentos de dispensación ambulatoria. No se encontraron diferencias estadísticamente significativas en la frecuentación de pacientes con coinfección COVID-VIH respecto al resto (1.172,76 ingresos/100.000 habitantes vs. 1.424,29, p=0,401), ni en su mortalidad (11,54 vs. 12,96%, p=0,939). El porcentaje de PCV con carga viral>50 copias fue similar antes y después de la pandemia (1,20% prepandemia vs. 0,51% en 2020, p=0,078).
ConclusiónNuestros resultados revelan que las estrategias implementadas durante los 8 primeros meses de pandemia han evitado el deterioro en parámetros de control y seguimiento empleados habitualmente en PCV. Además, contribuyen a la reflexión sobre el encaje de la telemedicina y telefarmacia en modelos asistenciales futuros.
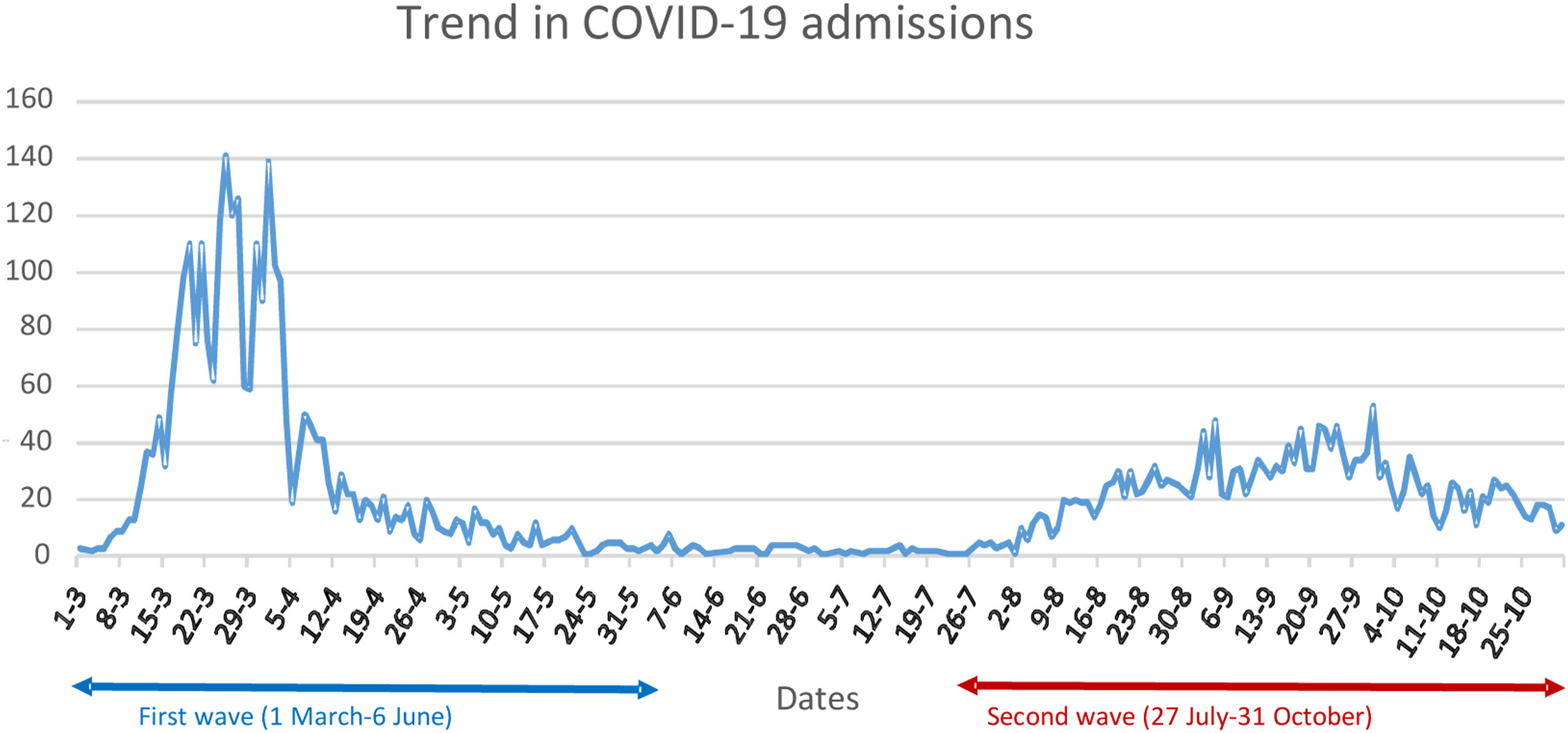
Since the first cases of COVID-19 infection were reported in Wuhan, China, between December 2019 and January 2020, the virus has spread swiftly around the world, with over 180 million infections and around four million deaths.1 To date, Spain has experienced four major waves, two of them between March and October 2020, portraying different characteristics, with the first, whose incidence reached a peak in March–April 2020, having a greater intensity and hospital impact but shorter duration than the second (which began in August of the same year).2
In this context, with all three facets of healthcare systems – clinical, research and management – targeted at the novel coronavirus, care of patients with other diseases may have been impaired in the process. Indeed, there have been theories about the arrival of a new wave, in the shape of the reappearance of acute and chronic cases postponed in these months.3 For instance, there is abundant evidence on the deterioration of care during the first two waves in clinical conditions such as diabetes4 or cancer.5
Research done on COVID-19 in patients with HIV infection (PWH) indicates that this group is especially vulnerable in relation to the pandemic, with their treatment possibly being affected and research resources being reallocated to COVID-19.6 In April 2020, in the midst of the first wave in Europe, the alert was raised regarding these patients’ apprehension at the possibility of becoming infected, about how the infection might affect them, and whether confinement and the saturation of hospitals would hinder access to their antiretroviral therapy (ART).7 As early as then, alternatives to hospital dispensing were proposed, such as delivery and follow-up by various organisations or dispatch and delivery by courier service.8 Furthermore, it was suggested that telemedicine services, with which PWH had already shown high levels of satisfaction,9 could fill the gap caused by the impossibility of in-person care.
Many studies that looked at the impact of the pandemic on PWH obtained conflicting results.10 Investigations on hospitalised patients suggest that co-infection with HIV does not significantly worsen the prognosis of COVID-19, increasing neither the risk of admission to ICUs nor mortality.11 However, a population-based study found that the risk of death from COVID-19 was higher in patients with co-infection.12 Most experiences agree on the worsening of adherence to treatment due to difficult access to drugs.10 When limited to a cohort of PWH, patients whose ART was the emtricitabine/tenofovir combination registered an incidence of COVID-19, hospitalisation and mortality lower than that of patients treated with other ARTs.13
To our knowledge, most published studies date back to the first wave of the pandemic, thus rendering them incapable of ascertaining the mid- and long-term impact of COVID-19 on the management of PWH. Accordingly, the aim of this study was to ascertain the healthcare outcomes of this group of patients in a region which experienced two major epidemic waves between March and October 2020 and recorded one of the highest cumulative incidences in Europe.
MethodsStudy and intervention designWe conducted an observational, retrospective, pre-post intervention study on patients attended at the HIV Infection Unit (HIVU) of the “12 de Octubre” University Hospital (H12O), with the dual objective of describing the characteristics of SARS-CoV-2 infection in this group of patients and evaluating the effectiveness of an intervention during the first two waves of the pandemic. “Intervention” was defined as the set of organisational measures implemented to mitigate risk of COVID-19 by limiting patients’ contact with the hospital as far as possible, and consisted of the home delivery of medications prescribed and dispensed to ambulatory patients undergoing follow-up at the HIVU, and preferential use of non face-to-face consultations.
Study setting and data-sourcesThe H12O is a 1300-bed, national and international referral hospital, and is where more than 6000 professionals work, providing specialised care to a health catchment area of approximately 450,000 inhabitants. As a member of the Spanish AIDS Research Network (Red de Investigación de SIDA/RIS) and other international research networks, the HIVU takes a leading role in pursuing the principal lines of research in the clinical area of PWH.
The PWH cohort undergoing follow-up at the HIVU was identified, by selecting patients who were attended at the HIVU from 2016 to 2020. In order to avoid possible seasonal variations and ensure the use of homogeneous magnitudes, the pre-intervention period was defined as the average of the months of March to October from 2016 to 2019, and the post-intervention period (pandemic) was defined as the same range of months in 2020.
The cohort of patients with COVID-19 was identified on the basis of alerts implemented in electronic health records (EHRs) at the onset of the pandemic, in line with World Health Organisation (WHO) specifications,14 adapted to the context of the H12O. Four main groups were identified: (i) patients with COVID-19 confirmed by diagnostic test (International Classification of Diseases, Tenth Revision (ICD-10) code U07.1); (ii) patients diagnosed with COVID-19 without a diagnostic test (ICD-10 U07.2); (iii) patients under investigation (suspected COVID-19); and (iv) patients with COVID-19 ruled out, apart from others such as those resulting from evaluation of respiratory risk or identification of institutionalised elderly in nursing homes situated in the H12O health catchment area (Table 1). These case profiles, which were modelled along with 58 observable clinical entities linked to management of patients with COVID-19 according to the ISO 13606 standard, are semantically linked to standard clinical terminologies, such as the Systematized Nomenclature of Medicine-Clinical Terms (SNOMED CT) (and its Spanish version) or the Logical Observation Identifiers Names and Codes (LOINC), with data being stored using a twin code-value structure which maintains their original meaning unaltered. This enables these data to be used as a support for clinical practice (primary use), as well as secondary uses, such as evaluation of healthcare quality, clinical management or research.15
Set of standard case profiles for identification of HIV and COVID-19 patient cohorts.
| Case profile | Standard | Code | Description |
|---|---|---|---|
| PWH | SNOMED CT | 86406008 | Human immunodeficiency virus infection |
| COVID-19 confirmed | SNOMED CT | 63681000122103 | Diagnosis of disease due to novel 2019 coronavirus, with specific diagnostic tests |
| COVID-19 diagnosed | SNOMED CT | 62811000122102 | Diagnosis of disease due to novel 2019 coronavirus, without specific diagnostic tests |
| COVID-19 under investigation | SNOMED CT | 840544004 | Suspected disease due to novel 2019 coronavirus |
| COVID-19 ruled out | SNOMED CT | 688232241000119100 | Diagnosis of disease due to novel 2019 coronavirus, ruled out |
| Patient at respiratory risk | SNOMED CT | 704296008 | At risk of impaired respiratory-system function |
| Patient in residential care | SNOMED CT | 160734000 | Lives in a nursing home |
Lastly, ambulatory dispensing data on medications prescribed to PWH during the pandemic were sourced from the H12O Pharmacy Department Data System.
Study variablesTo quantify the impact of the COVID-19 pandemic on the H12O, we considered patients admitted with a positive polymerase chain reaction (PCR) test result or clinical diagnosis of COVID-19. As variables indicative of HIVU healthcare activity, we analysed the number of outpatient visits in their different forms, and orders for determination of CD4 lymphocyte counts and HIV viral load in patients undergoing follow-up at the unit during the study periods; and, as outcome variables, we analysed the number of times that cohort patients required hospital admission or emergency care, and the proportion of these with a viral load result of, at least, >50copies during the periods considered.
Statistical analysisWe performed a descriptive analysis of the study variables, with discrete variables being expressed as frequencies and percentages, and quantitative variables as means and standard deviations (SDs).
Mean emergency visits, hospital admissions, and CD4 lymphocyte count and viral load orders were calculated per patient and per patient/month of exposure, taking into account, in each case, the start and end dates of the respective patient's follow-up at the HIVU, and the equivalent months corresponding to each of the periods studied.
We used the χ2 test or Fisher's exact test to compare discrete variables and the Student-t test or Mann–Whitney U test for the quantitative variables, as required. Statistical significance was defined as a p-value<0.05, and all analyses were performed using the Epidat v3.1 and SPSS v21 computer software packages.
ResultsThe study identified 3210 patients with an episode of HIV infection recorded in EHRs, 2760 (85.98%) of whom were attended at the HIVU from 1 January 2016 to 30 October 2020. Their mean age was 48.65 (11.58) years, and 670 (24.27%) were women. The mean follow-up period at the HIVU was 71.46 (36.06) months, and by October 2020, 129 (4.67%) patients had died. The sub-cohort attended in 2019 and 2020 included 2217 (80.33%) patients. During the pandemic, 5285 patients were admitted to the H12O due to COVID-19 (Fig. 1), with an admission rate among patients aged over 15 years of 1424.29 admissions per 100,000 population in the H12O referral area, and a crude intra-hospital mortality rate (CIMR) of 12.96%. Of these, 26 (0.49%) were PWH being followed up at the HIVU, and no statistically significant differences were found in terms of their admission rate (1172.76) or CIMR (11.54%) (p=0.401 and p=0.939 respectively).
HIVU activity did not vary significantly during the pandemic, with a monthly mean of 1700.38 (419.26) medical visits with respect to the mean for the same period from 2016 to 2019 (2100.06 (515.03), p=0.111), which means 400 fewer visits per month; while first and successive medical visits decreased, results appointments increased and there was a monthly mean of 106.87 (148.92) telephone consultations in 2020, which replaced traditional medical visits during the pandemic in order to reduce patient contact with the H12O as far as possible. Between March and October 2020, there were 2211 cancellations and non-attendances, detailed in Table 2. The first wave of the pandemic saw the introduction of home delivery of drugs dispensed to ambulatory patients, which accounted for 2075 (20.91%) of the 9924 overall ambulatory medications dispensed at Pharmacy in the period from 31 March to 18 June 2020. From this date, the face-to-face delivery of drugs was recovered, reinforcing preventive measures to avoid transmission.
Monthly trend in ambulatory activity, cancellations and non-attendances at the HIV unit (from 1 March to 31 October each year).
| Ambulatory activity | Cancellations and non-attendances | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average 2016–2019 | 2020 | p-Value | Average 2016–2019 | 2020 | p-Value | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| First medical visits | 18.22 | 8.23 | 9.75 | 4.62 | 0.024 | 12.06 | 5.83 | 10.37 | 5.70 | 0.586 |
| Successive medical visits | 588.5 | 154.78 | 337.75 | 99.36 | 0.002 | 69.72 | 21.75 | 122.88 | 97.97 | 0.174 |
| First nursing medical visits | 12.53 | 3.7 | 10.5 | 5.93 | 0.425 | 2.66 | 1.36 | 1.75 | 2.12 | 0.327 |
| Successive nursing medical visits | 592.13 | 127.98 | 448 | 184.68 | 0.091 | 93.63 | 23.28 | 139.0 | 88.0 | 0.196 |
| Results appointments | 100.87 | 40.46 | 209.13 | 90.5 | 0.012 | 9.21 | 4.86 | 1.38 | 1.3 | 0.002 |
| Non face-to-face consultations (*) | 787.81 | 219.37 | 578.38 | 175.94 | 0.054 | 2.31 | 1.81 | 1 | 1.19 | 0.113 |
| Telephone consultations | – | – | 106.87 | 148.92 | – | – | – | – | – | – |
| Total | 2100.06 | 515.03 | 1700.38 | 419.26 | 0.111 | 189.60 | 54.42 | 276.38 | 191.48 | 0.252 |
SD: standard deviation. (*) Non face-to-face consultations are appointments to evaluate results in which there is no need for the patient's presence (e.g., evaluation of intermediate analytical controls between 2 successive in-person visits). Only if there is some anomalous result that requires intervention, is an in-person appointment generated with the patient (this generally gives rise to a “classic” results appointment or a telephone consultation).
Taking each patient's months of exposure into account, mean emergency visits and hospital admissions per patient/month did not vary significantly between the pre- and post-intervention periods (0.044 (0.097) vs. 0.050 (0.634), p=0.657; and 0.012 (0.062) vs. 0.015 (0.181), p=0.539, respectively), and neither did mean orders per patient/month for determination of HIV viral load (0.171 (0.152) vs. 0.148 (0.614), p=0.073) or CD4 lymphocyte counts (0.044 (0.097) vs. 0.050 (0.634), p=0.195) (Table 3).
Emergency care, hospital admissions, and orders for determination of HIV viral load and CD4 lymphocyte counts in the pre-and post-intervention periods (values relating to the months of March to October in the periods considered).
| 2016–2019 | 2020 | p-Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Emergency care (patient) | 0.299 | 0.599 | 0.257 | 0.804 | 0.038 |
| Emergency care (patient/months of exposure) | 0.044 | 0.097 | 0.050 | 0.634 | 0.657 |
| Hospitalisation admissions (patient) | 0.076 | 0.229 | 0.067 | 0.380 | 0.280 |
| Hospitalisation admissions (patient/months of exposure) | 0.012 | 0.062 | 0.015 | 0.181 | 0.539 |
| Viral load (patient) orders | 1.102 | 0.678 | 1.056 | 0.839 | 0.037 |
| Viral load (patient/months of exposure) orders | 0.171 | 0.152 | 0.148 | 0.614 | 0.073 |
| CD4 lymphocyte (patient) orders | 0.906 | 0.549 | 0.852 | 0.740 | 0.003 |
| CD4 lymphocyte (patient/months of exposure) orders | 0.044 | 0.097 | 0.050 | 0.634 | 0.195 |
SD: standard deviation.
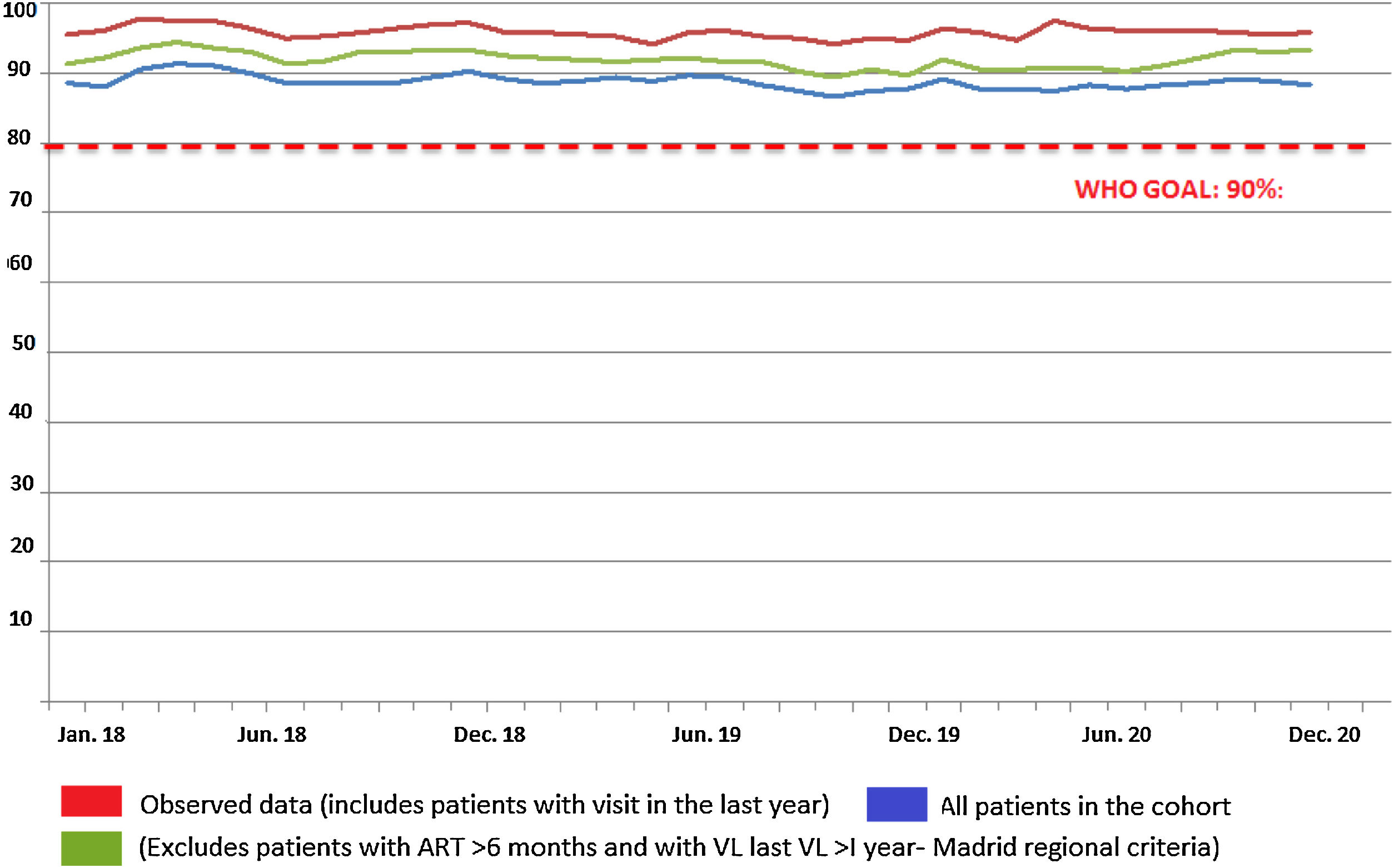
The monthly trend in the proportion of patients attended at the HIVU from 2018 to 2020 with viral load <50copies is shown in Fig. 2. Considering only the sub-cohort of patients attended in 2019 and 2020 for the purpose of comparing homogeneous magnitudes, no significant differences were seen between patients with viral load >50copies in the period March to October 2019 (22; 1.20%) and those in the same period in 2020 (6; 0.51%; p=0.078).
DiscussionWhile the COVID-19 pandemic has made it necessary to change the model of healthcare provided to PWH, our hospital data currently show no deterioration in the routinely used control and follow-up parameters.
The way in which healthcare institutions have addressed the COVID-19 health system overload, mitigating, as far as possible, the impact on the remaining diseases and healthcare processes, has been influenced by multiple factors. In the first place, the epidemiological situation has varied between geographical areas and over the course of the different waves. Accordingly, we think it is relevant to report the outcomes of a tertiary hospital in the central area of Spain, where, based on the overall prevalence of IgG antibodies, 18.6% of the population suffered from SARS-CoV-2 infection in 2020.16 Yet even within a given setting, and assuming circulation of the same virus, different health centres display varying results, from which it can be deduced that the specific strategies implemented by each hospital can modify outcomes in the management of other health problems. One clear example are the data furnished by the gynaecology and obstetrics departments of two Israeli hospitals, during the first wave of the pandemic. One of these reported an approximately 35% decrease in the number of visits and a 9.9% increase in the percentage of vaginal births, with a rise in patients presenting with hypertension and gestational diabetes, as compared to the same period in the previous year.17 In contrast, the other health centre found no significant differences in indications for referral or type of birth.18
Recommendations at the onset of the pandemic for management of PWH highlighted the need to maintain the regularity of medical visits and ensure adherence to the treatment, thereby preventing disease progression and the ensuing appearance of opportunistic infections.6,19 At the H12O, the logical decrease in in-person care was quantified as 405 fewer medical visits per month, which amounts to a fall of approximately 33% with respect to the period 2016–2019. The average of 107 telephone consultations conducted during the months studied in 2020 made it possible to alleviate this decline in care, by reducing the difference in the total number of medical consultations to 19%, with the comparison between periods lacking statistical significance. Even so, it should be noted that it is highly probable that there may have been underreporting of telephone consultations during the peaks of greatest healthcare activity, due to the fact that the healthcare overload considerably conditioned routine work procedures at the HIVU.
The results obtained open up the debate about new healthcare models. Previous studies show a high degree of satisfaction with telemedicine among PWH, with this being a safe, cost-effective alternative in the chronic management of such patients.20,21 Prominent among the benefits reported by PWH are privacy and the decrease in travel, but they also stress their concern about healthcare quality, the communication received, and data-protection.10,22 It is obvious that these new strategies cannot be extended to all patients or all healthcare processes. While the pandemic has taught us about some of the advantages of telemedicine, such as the capacity to minimise patients’ and health professionals’ risk of contagion,23 it has also clarified important limitations. Added to the impossibility of performing certain diagnostic and therapeutic procedures remotely, is the evident difficulty faced by especially vulnerable patients when seeking to access such resources, whether for social, economic or demographic reasons.6
Among the available alternatives for teleconsultation with dispensing of treatment,24 our health centre opted for home delivery of drugs dispensed by the Hospital Pharmacy Department, with this translating as over 2000 items dispensed across the months studied. Hence, despite the investment of the necessary resources, this ensured confidentiality, safety, universal access to medical drugs and their traceability. Moreover, management of home deliveries was centralised, without depending on the specific availability of different companies, associations or volunteers.8 Telepharmacy was not limited to ARTs and was common in both primary and hospital care. Remote access to drugs has been linked to better compliance with quarantines and confinements,25 with various studies reporting that this care modality does not significantly increase drug-related adverse events.26,27 In those cases wherein-person pharmaceutical care was maintained, preventive measures and protection of patients and health professionals were reinforced,24 with stress laid on the use of face masks, frequent hand hygiene, and temporal and physical distancing.28
By comparing indicators for March-October 2019 to those for the same months in 2020, we are able to state that the dual strategy implemented at the H120 (no medical visits in person and ambulatory dispensing of drugs) has proved effective. No significant differences between the two periods were observed in the proportion of patients with undetectable viral load (<50copies/ml), with percentages of close on 95%, the WHO's goal being to achieve an undetectable viral load in more than 90% of PWH. The same occurs when we analyse the HIVU's main activity indicators, with similar figures being recorded for orders for determination of viral load and CD4 lymphocyte counts.
It should be noted that the outcomes at the H12O are in line with those obtained by other institutions. If a study corresponding to the first wave of the pandemic reported that PWH were not observed to display a worse clinical course of COVID-19 or a higher mortality,11 then our study confirms that these premises were maintained when the study period was extended, with a CIMR of 11.54% in PWH and 12.96% in all hospitalised patients. Analysis of 77,590 PWH with PCR+ for COVID-19 from 60 Spanish hospitals yielded a mortality rate of 8%.13 This figure – one that is lower than that obtained at the H12O – may be due to the fact that 36% of the sample comprised cases with ambulatory follow-up, presumably with lower severity and mortality.
Our study has limitations. Despite having opted, in line with WHO guidelines, to use determination of viral load as the method for monitoring and evaluating therapeutic adherence to ART,29 we nevertheless feel that analysis of other parameters and outcomes would enhance the conclusions. Specifically, the inclusion of Patients Reported Outcomes (PROs) would make it possible to ascertain the health status and quality of life of PWH in relation to their health problem,30 and the incorporation of Patient Reported Experience Measures (PREMs) might respond to the question of whether aspects such as patient-perceived humanisation or quality of care were affected during these months. Despite the fact that the results do not show statistically differences in the viral load of the patients before and after the first two waves of the pandemic, there is no information on this parameter in the patients who did not go to the hospital (and who did not were tested). Furthermore, the observational and retrospective nature of the study, without random allocation of the interventions, shows the overall impact of the strategy implemented but does not allow for the magnitude of the effect of each measure to be separately ascertained. Lastly, the follow-up will have to be extended in order to ascertain long-term outcomes in PWH, the impact of possible successive waves, and the commencement of vaccination in this group of patients.
In conclusion, the outcomes obtained at the H12O show the effectiveness in the care of PWH of non face-to-face consultations and home delivery of drugs dispensed to ambulatory patients during the COVID-19 pandemic. The progressive recovery of in-person activity should be accompanied by the debate about how telemedicine and telepharmacy can fit into our healthcare systems in future.
Conflict of interestThe authors declare no conflicts of interest.
This work was funded by project PI18/01047, from the Instituto de Salud Carlos III and co-funded by the European Regional Development Fund, and approved by the Research Ethics Committee of our Institution.