Pre-exposure prophylaxis (PrEP) is a biomedical intervention aimed at preventing the transmission of the human immunodeficiency virus (HIV) in people at high risk of infection. Currently, the most frequent route of HIV transmission in Spain is sexual intercourse. It accounted for 83.1% of new HIV diagnoses in 2018, representing gay, bisexual and other men who have sex with men (MSM), the 56.4% of new diagnoses.1
The currently approved regimen by the European Medicines Agency (EMA) and suggested by the European AIDS Clinical Society (EACS) consists of co-formulated TDF/FTC3 1 tablet per day. The implementation of PrEP has shown a significant decrease in new HIV infections among vulnerable population.2
Although PrEP was approved by EMA several years ago, it became available in Spain in November 2019.3 The initial experience with our PrEP implementation has been recently published.4 PrEP is highly effective when adherence is high; however, rare cases of seroconversion may occur, mostly when adherence is poor. Resistance to antiretroviral therapy (ART), mostly to 3TC can occur, being most frequent when PrEP is initiated in extremely early cases where Ag/Ab test have been negative, or when the patient acquire infection between the screening period and PrEP initiation.5 We report two cases of HIV infection in PrEP users who developed resistance to ART.
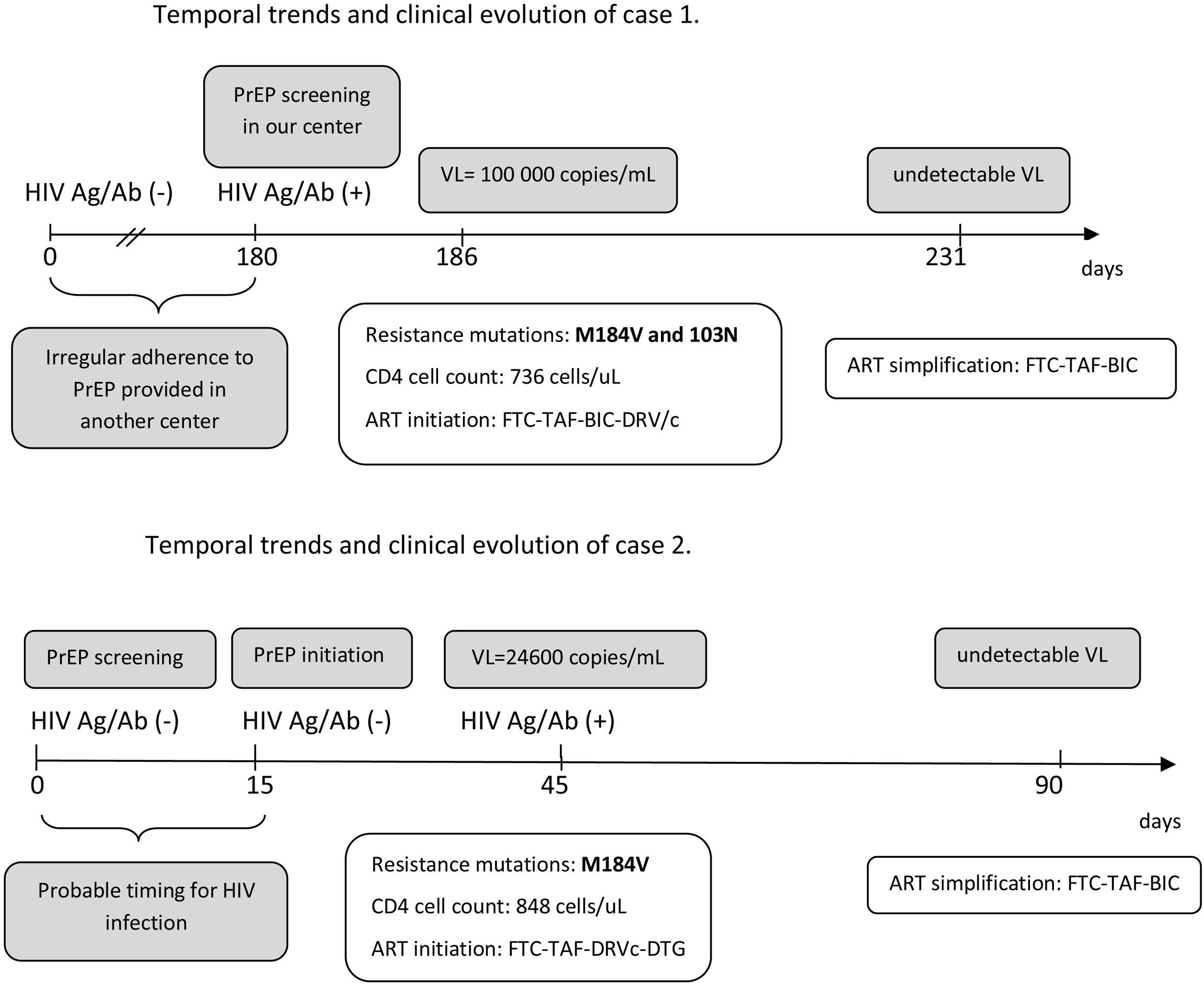
The first case is a 23-year-old male sex worker, who practiced chemsex and had been using PrEP for 6 months at another institution who was transferred to our center to continue PrEP In the baseline visit, positivity for HIV Ag/Ab was detected. Genotyping revealed mutations for M184V and K103N, suggesting exposure to a resistant strain. The patient reported use of condoms in more than 90% of cases but irregular adherence to PrEP. Fig. 1A shows the temporal evolution of the case.
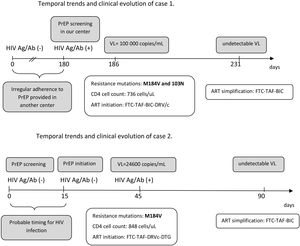
The second case is a 35-year-old male, who started the PrEP program with a first negative screen Ag/Ab test for HIV. At 15 days, a new determination for HIV (Ag/Ab rapid test) was performed prior to PrEP initiation. At one month PrEP visit, rapid Ag/Ab test was positive. Viral load revealed 24,600copies/mL, and the resistance genotyping test showed M184V and M184I substitution in a significant proportion (>90% of sequences for both substitutions together). Probably, the patient could have been infected within a few days before the time of the screening or between the time of screening and PrEP initiation. Fig. 1B shows the temporal evolution of the case.
The implementation of the PrEP in Spain represents a major progress in HIV prevention. However, many centers are still developing PrEP programs and real-life experience may be useful to prevent cases of seroconversion and development of resistances to ART that may limit treatment options.6 The two cases described emphasize the importance of baseline determinations prior to PrEP initiation, as well as the need for regular periodic controls as recommended in most PrEP protocols and guidelines.2,3 There are several scenarios for acquiring HIV infection and developing resistance in PrEP users. Exposure to a resistance strain seems to be the mechanism for the case 1 (although his adherence was also poor), while initiation of PrEP in an extremely early infection seems to be the mechanism for the second.
The period between initial screening in the program and PrEP initiation is critical and may drive to rapid development of resistance is HIV infection is unnoticed, as described by our second case. A molecular test (such as a PCR) could be considered for screening, but its cost limits this approach. In addition, the recommendations of condom use should be stressed in this period. As PrEP users seroconverting have frequently lower viral load compared to non-PrEP users, primary HIV infection is asymptomatic and, sometimes, seroconversion is delayed complicating diagnosis of infection.5 This more difficult diagnosis has been also described in the PrEP trials with cabotegravir long-acting7 and should always be considered when suspecting HIV infection in a PrEP user.
Overall, inconsistent adherence to PrEP often increases the likelihood of acquiring HIV infection in PrEP users.5 Therefore, it is extremely important to establish strategies to reinforce adherence to PrEP, based on patient's empowerment: complete information on PrEP, how to establish routines that adapt to their work and social life; strategies to avoid missing doses; monitoring of adherence at each visit.6 Real-life experience and challenging cases such as those described here might help centers where PrEP programs are being implemented.
DisclosureThe patients included in this letter have verbally consented to the inclusion of their case in this letter, always under the strictest anonymity.