The aim of this study is to review how did the first three COVID-19 waves affected the diagnostic of tuberculosis and to describe the extra-pulmonary Mycobacterium tuberculosis complex (TB) diagnosis.
Materials and methodsA retrospective observational study was done during the first three waves of pandemic to ascertain the impact on TB samples and to recover the extra-pulmonary TB cases we included the first two years of COVID-19. All relevant data was recovered from hospital and Clinical Microbiology records.
ResultsPrepandemic period showed an average of 44 samples per week for TB study; during the first three waves this number dropped to 23.1 per week. A reduction of 67.7% of pulmonary TB diagnosis was observed and an increase of 33.3% diagnosis of extra-pulmonary TB was noted when comparing pre-pandemic and pandemic period.
DiscussionThe number of declared cases and samples for TB diagnosis dropped during the first three COVID-19 waves due to the overstretched Public Health System which could lead to a delay in diagnosis, treatment and to the spread of TB disease in the general population. Surveillance programs should be reinforced to avoid this.
El objetivo de este estudio fue revisar cómo afectaron las primeras tres olas de la pandemia COVID-19 al diagnóstico de tuberculosis y describir el diagnóstico de las infecciones extrapulmonares causadas por Mycobacterium tuberculosis complex (TB).
Materiales y métodosSe realizó un estudio observacional y retrospectivo durante el periodo que incluye las tres primeras olas de la pandemia para valorar el impacto en las muestras de TB y para valorar el diagnóstico de las TB extrapulmonares se amplió el periodo de estudio para incluir los 2 primeros años de la COVID-19. Todos los datos relevantes se extrajeron de la base de datos del hospital y del Servicio de Microbiología y Parasitología Clínica.
ResultadosEn el periodo prepandémico se recibían una media de 44 muestras por semana para el estudio de TB; durante las tres primeras olas ese número cayó a 23,1 por semana. Se observó una reducción del 67,7% en el diagnóstico de la TB pulmonar y un aumento del 33,3% en el diagnóstico de la TB extrapulmonar cuando se comparó con los datos prepandemia.
DiscusiónEl número de casos declarados y el número de muestras para el diagnóstico de TB cayó durante las tres primeras olas del COVID-19 debido a la saturación del Sistema Nacional de Salud, lo que podría llevar a un retraso en el diagnóstico, tratamiento y a un aumento de la transmisión en la población general. Los sistemas de vigilancia deberían reforzarse para evitar esto.
SARS-CoV-2 is a novel virus that has spread throughout the world for more than two years. It started in China in December 2019 and was declared a pandemic by the World Health Organization (WHO) on the 11th of March 2020.1 Up to the end of July 2022 over 567 million cases have been confirmed and more than 6.3 million deaths have been reported globally.2 This virus can cause both, community, and hospital-acquired pneumonia. Mycobacterium tuberculosis complex is the most common cause of pulmonary tuberculosis (TB) with an estimate of 10 million cases and 1.3 million deaths annually.3 In Spain up to the year 2018, 4386 cases were reported; 3171 pulmonary TB and 1215 extra-pulmonary TB.4 According to the document “Strategy for Public Health 2022” published on August 2022 by the Health Ministry of Spain, the incidence of TB is 9.24 cases/100,000 residents.5 The impact of COVID-19 pandemic on tuberculosis control showed that case notifications have plummeted because of pandemic-related disruptions in services and for the first time in more a decade, tuberculosis mortality has increased according to WHO Global tuberculosis report 2021.3 The diversion of resources (including human and financial) away from routine services to manage the pandemic discouraging people from visiting TB services that made to fall the diagnosis and new treatments for TB. Mycobacterium tuberculosis complex is a respiratory microorganism known to affect humans and might share some of the clinical presentation symptoms with SARS-CoV-2 infection. As during the COVID-19 pandemic, several respiratory co-infections were reported, including fungal and bacterial pathogens6,7 co-infections of TB and SARS-CoV-2 infection may have occurred in the past two years. Due to this, clinicians should have a high index of suspicious that the two entities might coexist so there is no delay in diagnosing both. Up to 25% of pulmonary TB develops extra-pulmonary TB disease virtually infecting any organ so definitive diagnosis usually requires invasive procedures and image studies as symptoms can be very heterogeneous as described by Ramírez-Lapausa et al.8 This type of clinical presentation usually occurs when there are diagnostic delays from the onset of symptoms to isolation microbiological of TB.
The aim of this study is to review how did the first three COVID waves affected the microbiologic diagnostic of TB and to describe the extra-pulmonary Mycobacterium tuberculosis complex (TB) diagnosis done at our hospital during the pandemic period.
Material and methodsA retrospective observational study was done in the Clinical Microbiology and Parasitology Department at the University Hospital La Paz, a tertiary care centre in Madrid (Spain) with 1268 beds covering urban and rural areas. Two different time periods were selected. We included the year 2019 to see the average samples sent to out laboratory for the study of TB, comprising a total of 26 months which included the first three waves of the COVID-19 pandemic (January 2019 to February 2021). We reviewed the number of samples received to study tuberculosis infections and the number of positive results for COVID-19. To describe the extra-pulmonary TB cases, we extended the study period to comprise the first two years of COVID-19 pandemic in order to include more cases (February 2020–December 2021). All demographic, clinical and microbiological data were recovered from the Clinical Microbiology and Parasitology Department database and the electronic health records from our hospital.
All samples received for the study of TB infection were cultured in liquid medium (Mycobacteria Growth Indicator Tubes MGIT® Beckon Dickinson, Franklin Lakes, New Jersey, USA) and in Lowenstein-Jensen® solid medium (Beckton Dickinson, Franklin Lakes, New Jersey, USA). The incubation period for the MGIT® medium was 35 days and for the Lowenstein-Jensen® solid medium 56 days. All isolates from positive culture were identified by real time PCR (RT-PCR) (GeneXpert® Cepheid, Sunnyvale, California, USA) or PCR/reverse hybridization technique (GenoType Mycobacterium®, Bruker, Billerica, Massachusetts, USA). To undergo antibiotic susceptibility testing we performed the BBL™ MGIT™ AST SIRE Test, Beckon Dickinson (Franklin Lakes, New Jersey, USA) according to the manufacturer specifications and to test pyrazinamide all isolates were sent to the National Reference Centre for Microbiology (Majadahonda, Madrid). At the time, two commercial RT-PCRs were used indistinctly for the detection of SARS-CoV-2 in respiratory samples (TaqMan® 2019 nCoV assay Kit V1, Thermo-Scientific Inc. Franklin, MA, USA and SARS-CoV-2 RT-PCR Kit, Vircell S.L., Granada, Spain).
The study was approved by the Clinical Research Ethics Committee of Hospital Universitario La Paz with the code PI-5249.
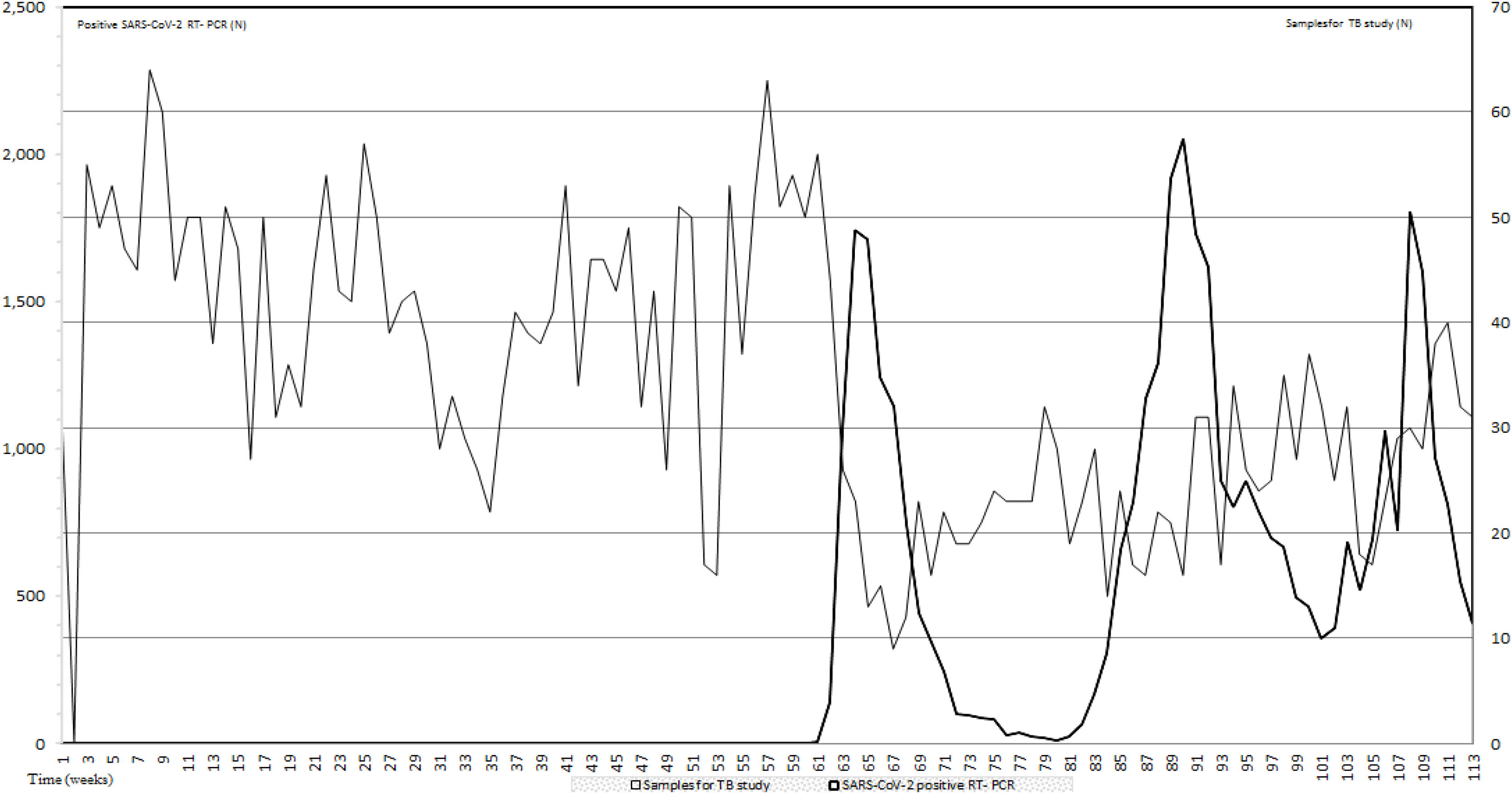
ResultsIn this study to assess the impact of the first three pandemic COVID-19 waves on TB diagnostic we compared the number of samples for TB infection studies and the RT-PCR positive results for COVID-19 during the first 26 months of the COVID-19 pandemic. During this period, an average of 33.97 samples per week for TB detection were received in our laboratory. Data prior to the appearance of COVID-19 from January 2019 to February 2020 when the first RT-PCR for SARS-CoV-2 was positive, showed an average of 44 samples per week. From the first to the third COVID-19 waves, samples per week for TB study dropped to 23.1 (47.5%) and 35.4 (19.55%) respectively when compared with prepandemic data. In between waves 24.5 samples per week were received. All data are included in Fig. 1.
Number of samples for TB study sent to the Clinical Microbiology and Parasitology Department (light grey) and number of SARS-CoV-2 RT-PCR (black). The period of study includes pre-pandemic data (January 2019–February 2021) and the period that comprises the first three COVID-19 waves; a total of 26 months.
During the pre-pandemic period, in our hospital we diagnosed 96 patients with pulmonary tuberculosis while the number of pulmonary tuberculosis during the study period dropped to sixty-five (a reduction of thirty-one patients; 67.7%). Regarding extra-pulmonary tuberculosis, during the pre-pandemic period, 21 patients were diagnosed while 28 patients with extra-pulmonary tuberculosis were diagnosed during the first three waves from the first two years of the COVID-19 pandemic (February 2020–December 2021). From these patients, 14 were female (50%). Thirteen patients were less than 50-year-old (46.43%), 8 were between 51 and 70 (28.57%) and seven had more than 70 year-old. Twelve patients (44.4%) were born in Spain while fifteen (55.5%) were immigrants. From these 15 patients, nine (60%) were from South America. We found that eight patients had neither underlying disease nor risk factors. The most presented risk factors were high blood pressure (HBP) and immunosuppression due to different causes. To diagnose extra-pulmonary TB, we recovered 16 nodal positive cultures, six osteoarticular samples, three urine samples, four digestive samples and one cerebral sample. Seven out of 28 patients had also positive respiratory samples for TB which is interesting in terms of transmission and control of the disease.
The time between the onset symptoms and the diagnosis had a median of eight weeks and an IQR was 18-4. Regarding image studies, ultrasonography was performed for the diagnosis of nodal, genitourinary and osteoarticular locations (in three patients). Chest radiography was the exclusive radiological diagnostic imaging in two patients. Combination of two or more radiological techniques was necessary in some patients with multiple sites of extrapulmonary disease (Table 1). Indirect study for TB (Mantoux or Interferon Gamma Release Assay-IGRA test; QuantiFERON Qiagen®, Hilden, Germany) was not performed in all patients. Fourteen patients had an IGRA test done (11 positive, two indeterminate and one negative) and seven patients had a Mantoux test performed all were positive. Four patients had both tests performed and both tests were positive.
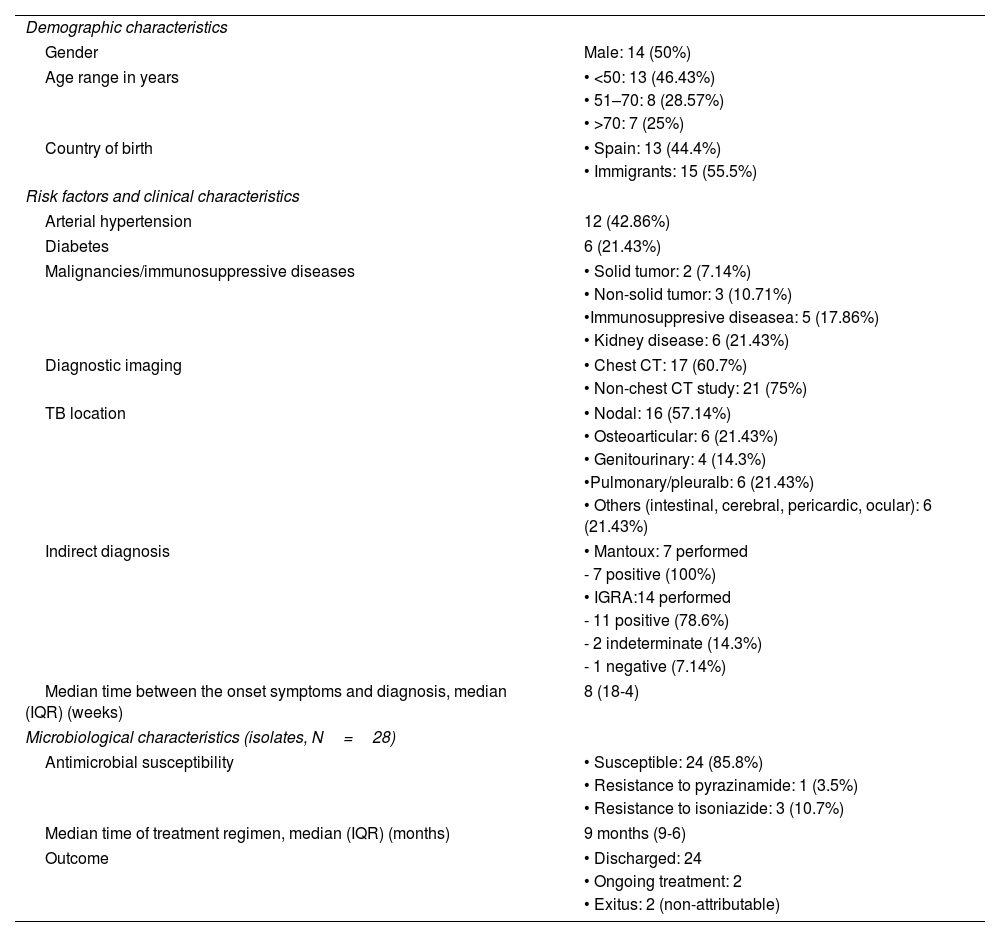
Demographic, clinical and microbiological characteristics of the patients with extra-pulmonary TB (N=28).
| Demographic characteristics | |
| Gender | Male: 14 (50%) |
| Age range in years | • <50: 13 (46.43%) |
| • 51–70: 8 (28.57%) | |
| • >70: 7 (25%) | |
| Country of birth | • Spain: 13 (44.4%) |
| • Immigrants: 15 (55.5%) | |
| Risk factors and clinical characteristics | |
| Arterial hypertension | 12 (42.86%) |
| Diabetes | 6 (21.43%) |
| Malignancies/immunosuppressive diseases | • Solid tumor: 2 (7.14%) |
| • Non-solid tumor: 3 (10.71%) | |
| •Immunosuppresive diseasea: 5 (17.86%) | |
| • Kidney disease: 6 (21.43%) | |
| Diagnostic imaging | • Chest CT: 17 (60.7%) |
| • Non-chest CT study: 21 (75%) | |
| TB location | • Nodal: 16 (57.14%) |
| • Osteoarticular: 6 (21.43%) | |
| • Genitourinary: 4 (14.3%) | |
| •Pulmonary/pleuralb: 6 (21.43%) | |
| • Others (intestinal, cerebral, pericardic, ocular): 6 (21.43%) | |
| Indirect diagnosis | • Mantoux: 7 performed |
| - 7 positive (100%) | |
| • IGRA:14 performed | |
| - 11 positive (78.6%) | |
| - 2 indeterminate (14.3%) | |
| - 1 negative (7.14%) | |
| Median time between the onset symptoms and diagnosis, median (IQR) (weeks) | 8 (18-4) |
| Microbiological characteristics (isolates, N=28) | |
| Antimicrobial susceptibility | • Susceptible: 24 (85.8%) |
| • Resistance to pyrazinamide: 1 (3.5%) | |
| • Resistance to isoniazide: 3 (10.7%) | |
| Median time of treatment regimen, median (IQR) (months) | 9 months (9-6) |
| Outcome | • Discharged: 24 |
| • Ongoing treatment: 2 | |
| • Exitus: 2 (non-attributable) | |
aRheumatoid arthritis, HIV and CD4+ lymphocytopenia.
bThese samples were obtained after extra-pulmonary TBC was diagnosed.
Twenty-five isolates were sensible to all antituberculous drugs. During the prepandemic period no antituberculous drug resistance was observed. During the time of study, 3 TB isolates (10.7%) presented resistance to isoniazide and just one case (3.5%) to pirazinamide (Mycobacterium bovis). Treatments regimens were adjusted to the location and to the sensibility profile. The median treatment time was 9 months (IQR 9-6). Twenty-four patients were already discharged, two patients were undergoing treatment at the time of the study and two patients died from not-attributable causes.
DiscussionWe noticed a reduction in the number of samples for the study of TB during the first three COVID-19 waves when compared with the number of positive RT-PCR in our hospital and compared with pre-pandemic 2019 period.
A decrease in TB cases detection in 2020 by 18% globally and by up to 24% in high TB burden countries due to the impact of the COVID-19 pandemic has been observed in the National Tuberculosis Surveillance Programme services.3,9 As Jinsoo Min et al. suggested, this could be due to reassignment of health staff in an overstretched health services during COVID-19, reduced patients referrals and social coughing becoming an stigma.10 In Italy during the first 3 months of lockdown Francesco Di Gennaro et al. described similar findings.11 However, data regarding Australia reflects a slight increase of TB notifications during this period.12 This could be explained by the fact that the lockout practiced in that country was a very effective one enabling them to control COVID-19 outbreaks, which avoided overstretching their Public Health System.13 The Global Tuberculosis Report 2021,3 estimates that the COVID-19 pandemic has reversed almost 10 years of progress in providing essential TB services and reducing the TB disease burden worldwide. The number of new declared cases has shown an important decrease resulting in an increase in TB deaths especially in low-income countries. Indeed, in October 2020 it was calculated that the effect of the 3-month lockdown would result in an excess cost from 4.1% to 7.9% over the next five years.14
During the pandemic period, a reduction of pulmonary tuberculosis was observed in our hospital when compared with pre-pandemic period. On the other hand, regarding extra-pulmonary tuberculosis, a slight increase was observed in the same period. The overlapping symptoms in pulmonary TB with COVID-19 disease symptoms could have been a bias in suspecting TB pulmonary disease and might have been also a reason beside the reduction of samples collected for TB study for the reduction in pulmonary TB diagnostic and could also explain the slight increase diagnosis in extra-pulmonary TB diagnostics during this period.
Yu Pang et al.15 described the epidemiology of extra-pulmonary TB among inpatients in China concluding that the most frequent form for extra-pulmonary TB were osteoarticular and pleural presentation. Most of these diagnoses were done from clinical symptoms suggesting a high-risk likelihood of diagnostic delays and misdiagnosis of extra-pulmonary TB cases.
One of the limitations of this study is that the onset time of extra-pulmonary disease can be long and variable depending on multiple factor so the impact of COVID-19 pandemic is difficult to ascertain. The main limitation of our study is that it is a single-centre study, and the number of patients is low. Further multicentre studies addressing larger cohorts in other regions would clarify more the problem of TB diagnostics during the COVID-19 pandemic.
In conclusion, SARS-CoV-2 and TB share some of the onset clinical symptoms. In our centre we have seen a marked decrease of samples to study TB disease coinciding with the first three COVID-19 waves. An important reduction of pulmonary TB disease had also been reported while extra-pulmonary TB had slightly increased during this pandemic. All these variables overstretching National Health Systems and disrupting National Tuberculosis Surveillance Programs Services could suggest that these programs should be prepared for a possible rebound in TB cases after the pandemic due to the delayed and missed diagnoses which could result in an increased transmission as patients remain infectious for longer periods of time. Reinforcing these programs should be a priority to minimize the extra burden created worldwide on TB disease.
Authors’ contributionsM.R.B. has contributed in conceptualization, data curation, formal analysis, investigation, methodology, resources, writing, supervision, visualization and validation.
I.F.R. has contributed in data curation, formal analysis, methodology, supervision and visualization.
B.D.P. has contributed in conceptualization, investigation, methodology, writing, supervision, visualization and validation.
C.T.R. has contributed in conceptualization, writing, supervision, visualization and validation.
J.G.R. has contributed in supervision, visualization and validation.
FundingThe authors declare no financial support.
Conflicts of interestNone declared.
María Dolores Montero-Vega, María Pilar Romero†, Silvia García-Bujalance, Emilio Cendejas-Bueno, Carlos Toro-Rueda, Guillermo Ruiz-Carrascoso, Inmaculada Quiles-Melero, Fernando Lázaro Perona, Jesús Mingorance, Iker Falces-Romero, Almudena Gutiérrez-Arroyo, Iván Bloise, Gladys Virginia Guédez-López, Paloma García-Clemente, María Gracia Liras Hernández, Consuelo García-Sánchez, Miguel Sánchez-Castellano, Sol San José-Villar, Jorge Ligero, David Grandioso, Julio García-Rodríguez.