We aimed to describe antimicrobial use (AU) and quality of prescriptions (QP) in a 28-bed medical-surgical PICU of a European referral children's hospital during 2019.
MethodsAU data were expressed as days-of-therapy (DOT) over 100 days-present (DP) and as length-of-treatment (LOT). QP was based on monthly cross-sectional point-prevalence surveys. Length-of-stay (LOS), readmission rates (RR), and mortality rates (MR) were also collected.
ResultsPICU AU accounted for 13.5% of the global hospital AU; the median PICU density of AU was 1.4 (IQR 1.3–1.5) times higher than that of the rest of the hospital areas. Antibacterials represented 88.5% of the overall AU, cefazolin and amoxicillin-clavulanate being the most used drugs. A high QP rate was observed (149/168 optimal, 88.9%), with room for improvement in prophylactic regimens and de-escalation of broad-spectrum regimens. LOT, LOS, RR, and MR remained stable.
ConclusionsPICU AU represented a major portion of the global hospital AU. Despite high QP rates, prophylactic and broad-spectrum antibiotic regimens were optimizable.
Se describe el uso de antimicrobianos (AU) y la calidad de las prescripciones (QP) durante 2019 de una UCI pediátrica médico-quirúrgica de 28 camas de un hospital infantil europeo de tercer nivel.
MétodosEl AU se expresó en días de tratamiento (DOT) por 100 días-presente (DP) y en duración de tratamiento (LOT). La QP se midió en cortes mensuales. Asimismo, se recogieron datos sobre duración de ingreso (LOS), tasas de reingreso (RR) y tasas de mortalidad (MR).
ResultadosEl AU de la UCI pediátrica representó el 13,5% del AU global del centro y la densidad media de AU fue 1,4 (RIC 1,3-1,5) veces mayor que la del resto de áreas hospitalarias. Los antibacterianos representaron el 88,5% del total de AU, siendo cefazolina y amoxicilina-clavulánico los fármacos más utilizados. Se observó una tasa elevada de prescripciones óptimas (149/168; 88,9%), con margen de mejora en las profilaxis y el desescalado de tratamientos de amplio espectro. LOT, LOS, RR y MR se mantuvieron estables.
ConclusionesLa UCI pediátrica representó una parte importante del AU global hospitalario. A pesar de la elevada QP global, los regímenes antibióticos profilácticos y de amplio espectro resultaron optimizables.
Antimicrobial use (AU) is high in the pediatric population, especially among pediatric intensive care unit (PICU) patients.1,2 PICU patients are at risk of healthcare-associated infections, many of which are caused by resistant microorganisms that require broad-spectrum antibiotics.2 PICUs also account for a high use of prophylactic antimicrobials.2,3 Antimicrobial stewardship programs (ASP) have proved their effectiveness in reducing AU in children,1,4,5 but data on the most effective interventions for children and, especially, for the critically ill pediatric population remain limited.1,4 Moreover, the use of classical AU measures in adults, such as “defined daily dose”, is discouraged in children because of the weight-based dosage of antimicrobials in this population.6,7
Data about the impact of PICU AU on the total hospital AU are lacking, although AU density could be up to three times higher than in ward units according to adult experience.8 Also, very few studies have reported on ASP experiences in improving PICU quality of prescriptions (QP).4
We aimed to describe AU and QP in a referral pediatric PICU and their impact on total hospital AU during the year 2019.
MethodsSettingWe conducted a prospective observational study during 2019 in the 28-bed medical-surgical PICU of Hospital Sant Joan de Déu (Barcelona, Spain), a 268-pediatric-bed referral hospital with a full range of medical and surgical specialties and a 4-bed bone marrow transplant unit. The yearly number of hospital and PICU discharges are around 15,000 and 1200, respectively; among the latter, approximately 45% correspond to surgical patients (neurosurgery and cardiac surgery, one third each). A median of 6 extracorporeal membrane oxygenation procedures are performed yearly. The median Pediatric Risk of Mortality III score (PRISM III) for PICU-admitted patients in 2019 was 3.0 (IQR 2.8–3.6). The fully operational hospital ASP was implemented in January 2017. The main ASP strategy was face-to-face and/or electronic post-prescription review with feedback, together with other non-restrictive and educational strategies (see Suppl. Appendix 1).5 This study was approved by the local ethics committee (ref. 32–20), which granted a waiver of individual informed consent. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
DesignData were collected from the e-prescription system and electronic clinical charts between January 1 and December 31, 2019. AU was calculated using days-of-therapy (DOT), defined as the aggregate sum of days that a specific antimicrobial was administered to a patient.5,9 To express density of AU, DOT was standardized to 100 days present (DP), considering one day present any amount of time in a calendar day spent by a patient in a definite hospital location.5 Length-of-therapy (LOT) was used as a complementary AU indicator and defined as the duration of antimicrobial treatment in days. All systemic antibacterials, antifungals, and antivirals were included. Other complementary measures included length-of-stay (LOS), PICU readmission rates during the hospital admission (RR) and mortality rates within PICU admission (MR).
QP was recorded based on monthly cross-sectional point-prevalence surveys (PPS) during which all the prescriptions for PICU patients were evaluated. For a prescription to be considered ‘optimal’ the prescribed drug should follow local reference guidelines accounting for patient allergies, and should be administered through the correct route, dose, and schedule, and for an appropriate duration (considering the actual duration or the expected duration recorded in the e-prescription system or in the patient medical record; Suppl. Appendix 1).5
Statistical analysisCategorical variables were reported as proportions with 95% confidence intervals, and continuous variables as medians with interquartile ranges (IQRs). Correlation between variables was calculated using Pearson's correlation coefficient. Statistical analysis was carried out using SPSS version 25.0 software (IBM Corp., Armonk, NY, U.S.A.). Statistical significance was defined as a p-value<0.05.
ResultsDuring 2019, 966 out of 1302 (74.2%) patients admitted in the PICU received antimicrobials. The PICU AU represented 13.5% of the global hospital AU during 2019 (PICU and all hospital areas absolute DOT: 6827 and 50,666, respectively). PICU presented a median density of AU 1.4 (IQR 1.3–1.5) times higher than that of the rest of hospital areas.
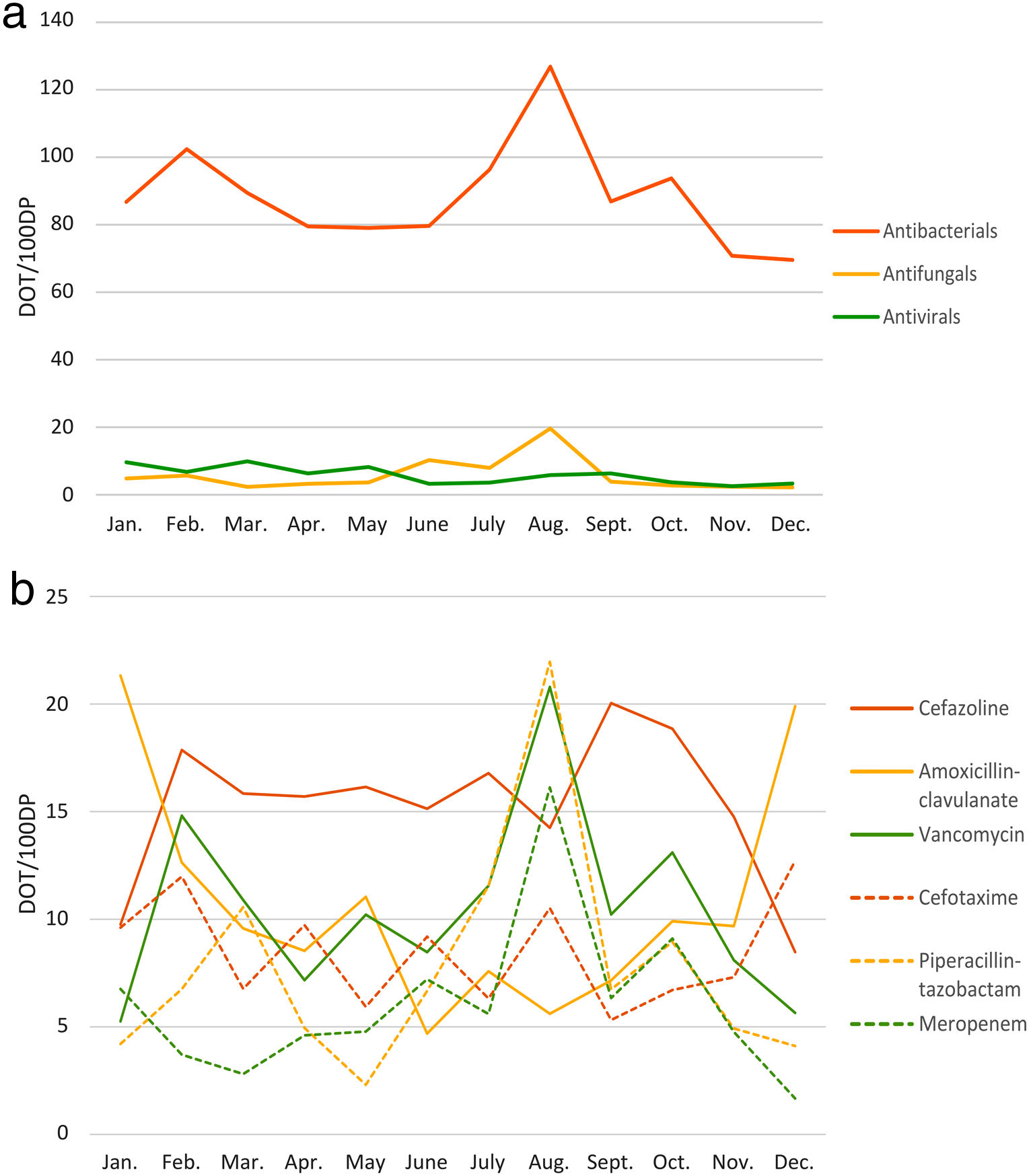
Of the annual PICU AU, 88.5% corresponded to antibacterials, 5.8% to antivirals, and 5.7% to antifungals (see Fig. 1 and Suppl. Table 1). The parenteral route prevailed (82.1%). Year 2019 most frequently used antibiotics were cefazolin (183.6 DOT/100DP, 15.3%), amoxicillin-clavulanate (127.6 DOT/100DP, 10.6%), vancomycin (126.2 DOT/100DP, 10.5%), and cefotaxime (102.1 DOT/100DP, 8.5%). Acyclovir (30.8 DOT/100DP, 2.6%) and liposomal amphotericin B (21.2 DOT/100DP, 1.8%) were the most commonly used antiviral and antifungal drugs, respectively. Main results expressed in monthly medians are summarized in Table 1.
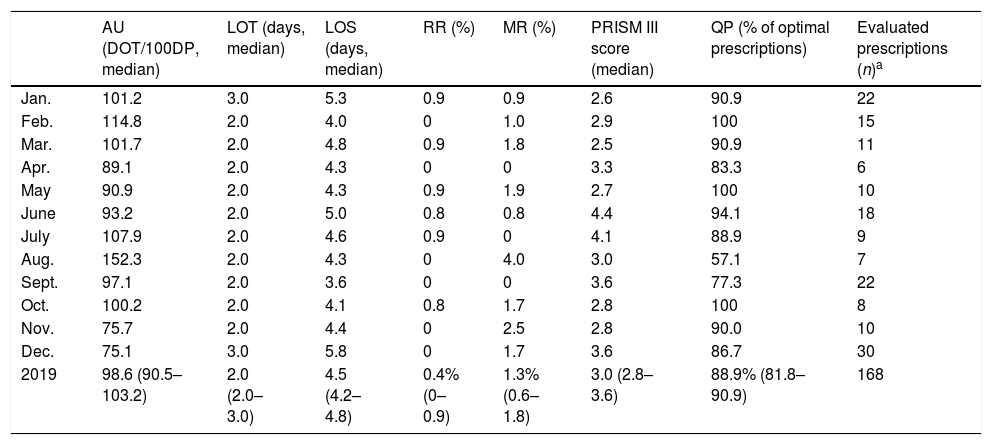
Pediatric intensive care unit antimicrobial use and complementary indicators during 2019. Monthly data are expressed as medians or proportions. Total 2019 data are expressed as medians and interquartile ranges of monthly values, or proportions and 95% confidence intervals of monthly values.
| AU (DOT/100DP, median) | LOT (days, median) | LOS (days, median) | RR (%) | MR (%) | PRISM III score (median) | QP (% of optimal prescriptions) | Evaluated prescriptions (n)a | |
|---|---|---|---|---|---|---|---|---|
| Jan. | 101.2 | 3.0 | 5.3 | 0.9 | 0.9 | 2.6 | 90.9 | 22 |
| Feb. | 114.8 | 2.0 | 4.0 | 0 | 1.0 | 2.9 | 100 | 15 |
| Mar. | 101.7 | 2.0 | 4.8 | 0.9 | 1.8 | 2.5 | 90.9 | 11 |
| Apr. | 89.1 | 2.0 | 4.3 | 0 | 0 | 3.3 | 83.3 | 6 |
| May | 90.9 | 2.0 | 4.3 | 0.9 | 1.9 | 2.7 | 100 | 10 |
| June | 93.2 | 2.0 | 5.0 | 0.8 | 0.8 | 4.4 | 94.1 | 18 |
| July | 107.9 | 2.0 | 4.6 | 0.9 | 0 | 4.1 | 88.9 | 9 |
| Aug. | 152.3 | 2.0 | 4.3 | 0 | 4.0 | 3.0 | 57.1 | 7 |
| Sept. | 97.1 | 2.0 | 3.6 | 0 | 0 | 3.6 | 77.3 | 22 |
| Oct. | 100.2 | 2.0 | 4.1 | 0.8 | 1.7 | 2.8 | 100 | 8 |
| Nov. | 75.7 | 2.0 | 4.4 | 0 | 2.5 | 2.8 | 90.0 | 10 |
| Dec. | 75.1 | 3.0 | 5.8 | 0 | 1.7 | 3.6 | 86.7 | 30 |
| 2019 | 98.6 (90.5–103.2) | 2.0 (2.0–3.0) | 4.5 (4.2–4.8) | 0.4% (0–0.9) | 1.3% (0.6–1.8) | 3.0 (2.8–3.6) | 88.9% (81.8–90.9) | 168 |
AU, antimicrobial use; DOT, days-of-therapy; DP, days-present; LOS, length-of-stay; LOT, length-of-treatment; MR, mortality rates; QP, quality of prescriptions; RR, readmission rates.
Regarding QP, 168 antimicrobial prescriptions for 95 patients were evaluated in the 12 monthly PPS, 20.8% of which had a prophylactic intention (see Suppl. Table 2). High rates of optimal prescriptions were observed in all PPS (median 88.9%, IQR 81.8–90.9) except in August (57.1%) (Table 1). The most frequent reasons for non-optimal prescription (19/168, 11.3%) were an inadequate spectrum (9/19, excessively broad and too narrow spectrum in 7 and 2, respectively) and excessive antimicrobial course duration (7/19). Nearly one third (30.0%) of non-optimal prescriptions had a prophylactic intention.
In August 2019, due to an increase in critically ill patients with hematological malignancies, meropenem, piperacillin-tazobactam, and vancomycin use peaked, together with an increase in LOS and MR and a marked decrease in QP (see Fig. 1). Other than this, LOT, LOS, RR, and MR remained stable during 2019 (Table 1). DOT and QP rates inversely correlated (Pearson's correlation; r=−0.565, p=0.056); no other associations were observed.
DiscussionAs previously reported,9 almost 75% of PICU-admitted patients in our study received at least one antimicrobial. PICU AU represented around 13% of total hospital AU, with a density of AU 1.4 times higher than that of the rest of hospital areas. Data evaluating the impact of PICU AU on total hospital AU are lacking.4 Most pediatric studies report either global hospital AU, or PICU AU alone.2–4 Our density of AU is lower than that in adult ICUs,8 which has been reported to be 2.2–3 times higher than in non-ICU areas,8 but it still represents a major portion of total hospital AU. This highlights how important it is for ASP to work together with PICU teams.2 Studies on PICU AU are scarce and difficult to compare, mainly due to differences in the indicators used, levels of care, areas of specialization, and the local antimicrobial resistance patterns.3,9,10
Although many authors have recommended the use of more than one indicator when reporting AU data, most PICU studies are based on standardized DOT alone.4 DOT have intrinsic limitations such as overrepresentation of the use of >1 antimicrobial regardless of their spectrum.6 We used LOT as a complementary indicator to overcome DOT limitations. LOT gives a good picture of the overall length of antimicrobial prescriptions in a specific unit or center and, together with LOS, is usually easier to understand to prescribers than DOT.11 The short LOT we observed (median 2.0 days) was likely due to the elevated proportion of prophylactic cefazolin prescriptions.9
Cefazolin is known to be one of the commonest antimicrobials in PICUs.2,9 In accordance with previous studies, longer than required prophylaxis and high use of broad-spectrum antimicrobials were identified as the main causes of non-optimal prescription.10,12,13 A number of authors have demonstrated that ASP can successfully reduce the use of the latter, which in turn can have a relevant impact on antimicrobial resistance rates.5,9 In our study, piperacillin-tazobactam and meropenem represented 7.8% and 6.1% of total AU during 2019, respectively. Except for the August peak, these rates are lower than those reported by PICUs similar to ours, in terms of patients’ complexity and conditions treated, in the absence of ASP, which range from 14.3% to 31.5%.12,13
RR and MR remained low and stable during the study period14; suggesting that ASP interventions did not lead to increased harm.1,5,7 In August 2019, the rise in broad-spectrum AU, LOS, and MR was accompanied by a drop in the QP rate. This can be partly explained both by the increased complexity of the patients’ conditions, and a decline in the number of admissions after elective surgeries during the summer period. Permanent monitoring by ASP teams can help detect unexpected variations in ASP indicators, allowing for implementation of corrective measures.7
QP monitoring is time-consuming and further complicates standardization due to the different QP indicators available.1,13 However, it should be kept in mind that the main objective of ASP is to improve quality of care, above decreasing AU or expenditure.7,13 Compared to previous studies, our QP rates were high10,13; nevertheless, some areas for improvement were identified, such as shortening prophylactic courses and de-escalating broad-spectrum antimicrobial regimens. The former can be achieved by means of biomarker-based decision algorithms, tailoring the duration of antibiotics to the intraoperative findings, automated e-prescription filters for certain drugs, or the implementation of periodical rounds with infectious diseases specialists and pharmacists.15
The single-center observational design, the short study period, and the lack of previous data for comparison are obvious limitations of our study. ASP evaluations should be complemented with microbiological indicators (i.e. extended spectrum beta-lactamase and carbapenemase producing Enterobacteriaceae, multi-drug resistant Pseudomonas aeruginosa or methicillin resistant Staphylococcus aureus) in the search for changes in resistance patterns. Finally, PPS do not allow to draw conclusions about data outside the specified time frame.
In summary, our study shows the relevant impact of PICU AU on the global hospital AU, although the density of use was lower than that reported in adult ICUs. A high rate of optimal prescriptions was observed, but unnecessary broad-spectrum regimens and longer than required prophylaxis were identified. Available data on PICU AU are scarce and heterogeneous, making comparisons difficult to establish. Appropriate ASP indicators for PICU patients are needed to establish targets, assess the impact of interventions, and permit benchmarking.
FundingNo specific funding was received for this study; data were generated as part of the routine work of Hospital Sant Joan de Déu.
Conflicts of interestSílvia Simó Nebot was supported by “Contratos Río Hortega, Convocatoria 2018” (Acción Estratégica de Salud, Ayudas y Subvenciones, Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación, Spain) [CM18/00054]. Antoni Noguera-Julian was supported by “Subvencions per a la Intensificació de Facultatius Especialistes” (Departament de Salut de la Generalitat de Catalunya, Programa PERIS 2016–2020) [SLT008/18/00193].
Sílvia Simó Nebot and Clàudia Fortuny have received funds for speaking at symposia organized on behalf of Gilead Sciences.
The rest of the authors have no conflicts of interest to disclose.
We would like to thank Maria Ríos-Barnes, Manuel Monsonís and Mireia Urrea of Hospital Sant Joan de Déu’ Antimicrobial Stewardship Program, as well as Clàudia Fortuny and Franscisco José Cambra for their contribution to the study. We also thank all the Pharmacy Department and the Pediatric Intensive Care Unit team of Hospital Sant Joan de Déu for their daily dedication to improving the management and quality of care of critically ill pediatric patients and, finally, the computer, statistics, and hospital management teams of Hospital Sant Joan de Déu and Sant Joan de Déu Research Foundation for their invaluable help in antimicrobial use data extraction and analysis.