We report the activity of delafloxacin, a new fluoroquinolone with high affinity for both topoisomerase IV and DNA gyrase, against highly-levofloxacin-resistant invasive strains of Streptococcus pneumoniae.
MethodsA total of 173 highly-levofloxacin-resistant (MIC>32mg/L) S. pneumoniae invasive isolates were studied. The strains were isolated from blood (n=162) and other sterile fluids (n=11). Serotyping was performed by the Pneumotest-Latex and Quellung reaction.
Delafloxacin, levofloxacin, penicillin, cefotaxime, erythromycin and vancomycin MICs were determined by the gradient diffusion method following EUCAST guidelines and breakpoints.
ResultsAmong the isolates, 32.9% were penicillin non-susceptible, 19.7% cefotaxime non-susceptible, and 76.9% erythromycin resistant. All were susceptible to vancomycin. Delafloxacin MIC50 and MIC90 (mg/L) values were 0.064 and 0.12, respectively; 60% (15/25) of serotype 9V isolates showed delafloxacin MICs≥0.12mg/L.
ConclusionsDelafloxacin was very active against highly-levofloxacin-resistant invasive isolates of S. pneumoniae. Isolates belonging to serotype 9V showed higher delafloxacin MIC values.
Analizamos la actividad de delafloxacino, una fluoroquinolona con elevada afinidad por la topoisomerasa i.v. y la ADN girasa, frente a cepas invasivas de Streptococcus pneumoniae altamente resistentes a levofloxacino.
MétodosSe estudiaron 173 cepas con CMI de levofloxacino >32mg/l procedentes de sangre (n=162) y otros fluidos estériles (n=11). El serotipado se realizó mediante Pneumotest-Latex y test de Quellung.
Las CMI de delafloxacino, levofloxacino, penicilina, cefotaxima, eritromicina y vancomicina se determinaron mediante gradiente de difusión siguiendo las recomendaciones del EUCAST.
ResultadosEl 32,9% de las cepas fueron no-sensibles a penicilina, el 19,7% no-sensibles a cefotaxima y el 76,9% resistentes a eritromicina. Todas fueron sensibles a vancomicina. Las CMI50 y CMI90 de delafloxacino (mg/l) fueron 0,064 y 0,120, respectivamente; 60% (15/25) de cepas del serotipo 9V presentaron CMI de delafloxacino ≥0,12mg/l.
ConclusionesDelafloxacino fue muy activo frente a S. pneumoniae altamente resistente a levofloxacino. Los aislados del serotipo 9V presentaron mayores valores de CMI de delafloxacino.
Community-acquired bacterial pneumonia (CABP) is one of the main causes of hospital admission in adults worldwide, and Streptococcus penumoniae is the most common etiological agent.1S. pneumoniae CABP is also associated with larger hospitalization periods, failure of treatment and a high sanitary costs.2 Thus, the emergence of multidrug-resistant S. pneumoniae isolates non-covered by the 13-valent pneumococcal conjugate vaccine (PCV-13) causing invasive pneumococcal disease (IPD) is a cause of concern, being a major problem in treating pneumococcal infections. Furthermore, it has been estimated that there are around three cases of non-bacteremic pneumococcal pneumonia episodes for every invasive pneumococcal pneumonia case detected in adult patients.3 Delafloxacin is a new dual-targeting anionic fluoroquinolone equally potent against topoisomerase IV and DNA gyrase, which are essential enzymes for bacterial DNA replication.1 It is thereby considered as a broad-spectrum antibiotic that inhibits both Gram-positive and Gram-negative bacteria, including anaerobes, and atypical respiratory tract pathogens (Chlamydia spp. and Mycoplasma spp.).4 Delafloxacin has demonstrated high intracellular penetration into the lung compartment, it offers intravenous and oral administration, and has no significant effect on several markers of prolongation of Q and T electrocardiogram waves in preclinical studies.1,5 Unlike other fluoroquinolones which usually decrease their bactericidal action under acidic conditions, delafloxacin keeps a neutral form and enhances its bactericidal activity.6 A phase III study that compared delafloxacin with moxifloxacin in adults with CAPB reported that delafloxacin was effective, non-inferior to moxifloxacin, and well tolerated in monotherapy.7 Although it was initially approved for the treatment of acute bacterial skin and skin structure infections, its use may be considered a treatment option as monotherapy for CAPB in adults. In this study, we report the activity of delafloxacin against highly-levofloxacin-resistant invasive strains of S. pneumoniae.
MethodsBacterial isolates. One hundred seventy-three highly-levofloxacin-resistant (MIC>32mg/L) strains from a total of 6524 S. pneumoniae invasive isolates, received between March 2007 and June 2019 at the Public Health Regional Laboratory of Madrid (Spain), were studied. The strains were isolated from blood (n=162), pleural fluid (n=5), cerebrospinal fluid (n=3), ascitic fluid (n=2), and pericardial fluid (n=1). Isolates were identified on the basis of phenotypic characteristics (colony morphology, Gram stain, catalase test and optochin susceptibility). Capsular serotypes were determined by Pneumotest-Latex (Statens Serum Institut, Copenhagen, Denmark) and by Quellung reaction using commercial factor antisera (Statens Serum Institut, Copenhagen, Denmark).
Antimicrobial susceptibility testing. Delafloxacin, levofloxacin, penicillin, cefotaxime, erythromycin and vancomycin MICs were determined by the gradient diffusion method (Liofilchem, Italy [delafloxacin]); Etest® bioMerieux, France [other antimicrobials]). Streptococcus pneumoniae ATCC 49619 and Staphylococcus aureus ATCC 29213 were used as quality control strains. Interpretation of MIC results was performed following the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines8: levofloxacin≤0.001mg/L susceptible, and >2mg/L resistant; penicillin≤0.06mg/L susceptible, and >2mg/L resistant; cefotaxime≤0.5mg/L susceptible, and >2mg/L resistant; erythromycin≤0.25mg/L susceptible, and >0.5mg/L resistant, and vancomycin≤2mg/L susceptible, and >2mg/L resistant. No breakpoints for delafloxacin have been stablished by the EUCAST to date. For the estimation of results, delafloxacin MICs for the 50% and 90% of the isolates (MIC50 and MIC90) were calculated. The comparison of categorical variables was performed by using the chi-square test.
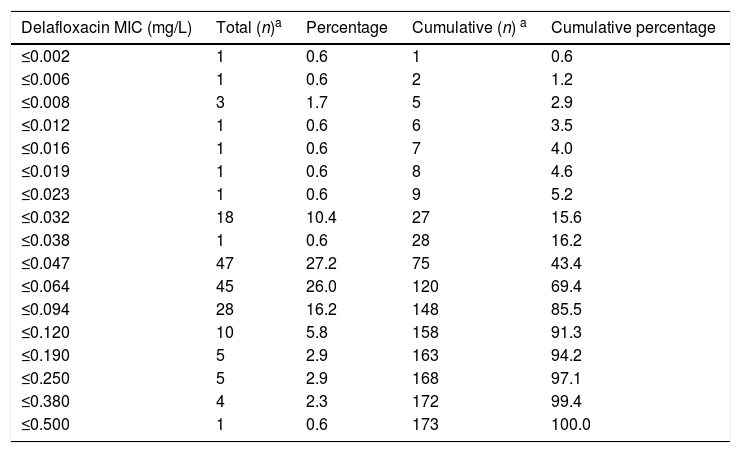
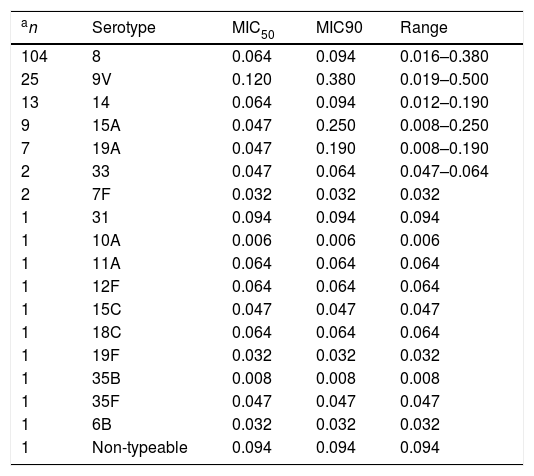
ResultsThe delafloxacin MIC50 and MIC90 (mg/L) values against the 173 levofloxacin-resistant isolates were 0.064 and 0.12, respectively (range≤0.002–0.5mg/L), with only one isolate showing a delafloxacin MIC of 0.5mg//L. The MIC distributions of delafloxacin against the pneumococcal strains are shown in Table 1. The distribution of serotypes/serogroups were: 8 (n=104), 9V (n=25), 14 (n=13), 15A (n=9), 19A (n=7), 7F (n=2), 33 (n=2), 31 (n=1), 6B (n=1), 10A (n=1), 11A (n=1), 12F (n=1), 15C (n=1), 18C (n=1), 19F (n=1), 35B (n=1), 35F (n=1), and non-typable (n=1), being 71.1% non-PCV-13 serotypes. Fifty-seven (32.9%) isolates were penicillin non-susceptible (MIC>0.06mg/L), 34 (19.7%) were cefotaxime non-susceptible (MIC>0.5mg/L), and 133 (76.9%) were erythromycin resistant (MIC>0.5mg/L). All isolates were susceptible to vancomycin. Results of MIC50 and MIC90 for delafloxacin against the different serotypes is shown in Table 2. Among isolates belonging to serotype 9V, 60% (15/25) had delafloxacin MIC values≥0.12mg/L, in comparison to 6.75% (10/148) of isolates belonging to the rest of serotypes (chi square test p<0.001). Only five isolates showed a delafloxacin MIC≥0.38mg/L, and four of them belonged to serotype 9V (chi square test p<0.001).
Antimicrobial activity of delafloxacin tested against highly levofloxacin-resistant (MIC>32mg/L) invasive Streptococcus pneumoniae.
| Delafloxacin MIC (mg/L) | Total (n)a | Percentage | Cumulative (n) a | Cumulative percentage |
|---|---|---|---|---|
| ≤0.002 | 1 | 0.6 | 1 | 0.6 |
| ≤0.006 | 1 | 0.6 | 2 | 1.2 |
| ≤0.008 | 3 | 1.7 | 5 | 2.9 |
| ≤0.012 | 1 | 0.6 | 6 | 3.5 |
| ≤0.016 | 1 | 0.6 | 7 | 4.0 |
| ≤0.019 | 1 | 0.6 | 8 | 4.6 |
| ≤0.023 | 1 | 0.6 | 9 | 5.2 |
| ≤0.032 | 18 | 10.4 | 27 | 15.6 |
| ≤0.038 | 1 | 0.6 | 28 | 16.2 |
| ≤0.047 | 47 | 27.2 | 75 | 43.4 |
| ≤0.064 | 45 | 26.0 | 120 | 69.4 |
| ≤0.094 | 28 | 16.2 | 148 | 85.5 |
| ≤0.120 | 10 | 5.8 | 158 | 91.3 |
| ≤0.190 | 5 | 2.9 | 163 | 94.2 |
| ≤0.250 | 5 | 2.9 | 168 | 97.1 |
| ≤0.380 | 4 | 2.3 | 172 | 99.4 |
| ≤0.500 | 1 | 0.6 | 173 | 100.0 |
Delafloxacin MIC50 and MIC90 and range (mg/L) according to different serotypes of highly levofloxacin-resistant (MIC>32mg/L) invasive Streptococcus pneumoniae.
| an | Serotype | MIC50 | MIC90 | Range |
|---|---|---|---|---|
| 104 | 8 | 0.064 | 0.094 | 0.016–0.380 |
| 25 | 9V | 0.120 | 0.380 | 0.019–0.500 |
| 13 | 14 | 0.064 | 0.094 | 0.012–0.190 |
| 9 | 15A | 0.047 | 0.250 | 0.008–0.250 |
| 7 | 19A | 0.047 | 0.190 | 0.008–0.190 |
| 2 | 33 | 0.047 | 0.064 | 0.047–0.064 |
| 2 | 7F | 0.032 | 0.032 | 0.032 |
| 1 | 31 | 0.094 | 0.094 | 0.094 |
| 1 | 10A | 0.006 | 0.006 | 0.006 |
| 1 | 11A | 0.064 | 0.064 | 0.064 |
| 1 | 12F | 0.064 | 0.064 | 0.064 |
| 1 | 15C | 0.047 | 0.047 | 0.047 |
| 1 | 18C | 0.064 | 0.064 | 0.064 |
| 1 | 19F | 0.032 | 0.032 | 0.032 |
| 1 | 35B | 0.008 | 0.008 | 0.008 |
| 1 | 35F | 0.047 | 0.047 | 0.047 |
| 1 | 6B | 0.032 | 0.032 | 0.032 |
| 1 | Non-typeable | 0.094 | 0.094 | 0.094 |
Fluoroquinolone resistance in pneumococci is due to changes in the fluoroquinolone binding site due to genetic mutations.9 Delafloxacin's dual targeting both to topoisomerase IV and to DNA gyrase could decrease resistance selection because it diminishes the likelihood of multiple mutational events in both enzymes.10 Our study includes a large series of invasive isolates of S. pneumoniae highly-resistant to levofloxacin in which the highest delafloxacin MIC was at least 128-fold lower than that of levofloxacin (0.5 versus>32mg/L). The high affinity of delafloxacin for the DNA gyrase could contribute to its lower MICs in comparison to levofloxacin.10 One limitation of our study is that we did not investigated possible mutations in parC and gyrA. Another limitation is that levofloxacin-susceptible isolates were not included in our study. This fact makes it impossible to know if delafloxacin MIC distributions are different between highly- levofloxacin-resistant isolates (with potential mutations in the quinolone resistance-determining regions [QRDRs] of the parC and/or gyrA genes) and levofloxacin-susceptible isolates (without mutations in the QRDRs). Previous studies have reported delafloxacin as a very active agent against S. pneumoniae isolates, being 128-fold (MIC50) and 64-fold (MIC90) more potent than levofloxacin, although they reported an increase in MIC values of delafloxacin when it was tested against levofloxacin resistant strains, resulting in a 16-fold (MIC50) and 32-fold (MIC90) increase, which indicates a possible cross-resistance event.4,11 This study shows that delafloxacin is a very active agent against invasive isolates of S. pneumoniae including highly-levofloxacin-resistant strains and confirms that even against these isolates it was at least >64-fold more potent than levofloxacin. To date, EUCAST has not yet established delafloxacin clinical breakpoints.8 In our study, many isolates showed multidrug resistance, and delafloxacin was equally active against penicillin-resistant, macrolide-resistant, and multiple-drug resistant S. pneumoniae as previously described.12 A high percentage of isolates included in our study (71.1%) were non-PCV-13 serotypes, indicating the need for new antimicrobials against these multi-resistant isolates. The potential benefit of the treatment with delafloxacin in adult patients with community acquired pneumonia has been reported, reaching microbiological success rates of 92.7% for S. pneumoniae and 87.5% for penicillin-resistant S. pneumoniae strains.12 The high proportion of serotype 8 levofloxacin-resistant strains included in our study was due to its previous wide prevalence of the 8-ST63 clone in our region.13 However, we have observed recently in our area in Madrid (Spain), a decrease in the fluoroquinolone resistance of this non-PCV-13 serotype.14
On the other hand, it is also important to note that serotype 9V, the second most frequent in our series, exhibited higher delafloxacin MIC levels, and this fact could be a cause for concern. In previous years, this resistant serotype belonged in our region mainly to the Spain9V-ST156 clone.13 The spread of this clone and other serotype 9V quinolone-resistant clones has been previously reported.15 Fortunately, the fact that the 9V serotype is included in the current 13-valent conjugate vaccine can limit its circulation in the community. In summary, our results show a high in vitro potency of delafloxacin against pneumococci, including highly-levofloxacin resistant and multi-drug resistant isolates. This fact, together with its equivalence of oral and intravenous formulations and its favourable safety profile, including the lack of corrected QT interval prolongation,6 support this new fluoroquinolone as a good addition to the therapeutic arsenal for the treatment of infections caused by multidrug resistant pneumococci.
Conflicts of interestThis is a fully independent study that has not received any financial support.