Introduction
Despite major advances in the medical arena, Staphylococcus aureus remains an important agent of infectious diseases in the human host. Its significance lies in its widespread existence and the broad spectrum of infections it can produce, ranging from inconsequential superficial skin infections to deep-seated life-threatening systemic infections 1. Indeed, some infections caused by S. aureus, namely bacteremia and endocarditis, are frequently associated with serious complications and high mortality rates 2-4. The emergence of antibiotic resistance has brought renewed attention to staphylococci 5. Methicillin-resistant S. aureus (MRSA) rates both in hospitalized and ambulatory patients have been escalating, and this resistant phenotype is now considered a major public health problem 6-8. Reduced susceptibility to other antimicrobials, including glycopeptides, is being increasingly recognized and further complicates the treatment of staphylococcal infections 9-11.
In this review, the authors report on the current trends in the epidemiology, diagnosis, clinical syndromes, and management of S. aureus infections in light of the organism's evolving antimicrobial resistance pattern.
Microbiology
Staphylococcus aureus belongs to the Micrococcaceae family. It is a nonmotile, non-spore forming, gram-positive coccus that may occur singly, or in pairs, short chains, or grape-like clusters. It is a facultative anaerobe, but grows better under aerobic than anaerobic conditions. The organism produces catalase and coagulase and grows readily on blood and chocolate agar. Colonies measure 1 to 3 mm and typically produce a yellow to golden pigment due to the presence of carotenoids. Most strains produce hemolysis within 24 to 36 hours on horse, sheep, or human blood agar plates 12.
Epidemiology
Worldwide epidemics of S. aureus disease have been recognized over the years 13,14. Outbreaks have been reported in a variety of settings, including hospitals 15, long-term care facilities 16 and outpatient clinics 17, as well as in the community 18.
Nosocomial Infections
Staphylococci have been long recognized as a problem on hospital wards, and the policy of routine ongoing surveillance for hospital-acquired staphylococcal disease is well justified 19-21. S. aureus is the leading cause of postoperative wound infection, and the second-most frequent cause of nosocomial pneumonia 22 and bacteremia 23. Together, S. aureus and coagulase-negative staphylococci account for 21% of the estimated 4 million infections acquired annually in United States hospitals 24. S. aureus nosocomial infections entail great expenditure. Over a two-year period from 2000 to 2001, the average cost of hospitalization in 994 US hospitals for patients with S. aureus infections was $48,834 compared to $14,141 for patients without such infections 21. In another study, the mean infection-related costs in patients with prosthetic devices and S. aureus bacteremia (SAB) amounted to $67,439 for hospital-acquired infections and $37,868 for community-acquired infections 25. In addition to the substantial economic burden, significant morbidity and mortality are associated with staphylococcal infections, particularly with invasive infections where mortality rates range between 19% and 34% 26,27.
Community-acquired infections
Staphylococcus aureus infections are commonly acquired outside the hospital, particularly among colonized individuals, and have been reported for several decades 28-30. However, the prevalence of infections caused by MRSA isolates has increased significantly. A Texas-based study in children noted a 14-fold increase in the rate of community-acquired MRSA infections in 2002 compared to previous years 31. Similarly among adults, the incidence of community-acquired staphylococcal infections varied from 29% in 1997 to 74% in 2002 32. In addition, recent studies have demonstrated a substantial increase in the rate of nasal colonization with MRSA in the community, from 0.8% in 2001 to 9.2% in 2004 33.
Nasal carriage
Staphylococcus aureus may be carried by normal people at various body sites without causing disease. This condition is referred to as colonization to distinguish it from actual infection. It should be noted, however, that colonization frequently precedes infection in susceptible patients 34. The anterior nares are the principal sites of colonization with three distinct patterns in the population: persistent carriers (20%), intermittent carriers (60%), or noncarriers (20%) 35. Whereas 10%-20% of healthy adults are persistently colonized with S. aureus, populations with higher colonization rates include patients with atopic dermatitis (up to 85%) 36, as well as surgical patients 37, hemodialysis patients 38, HIV-infected patients 39, and those with intravascular devices 40. Health care workers who come in contact with patients colonized or infected with S. aureus have higher rates of nasal carriage than providers without such contact 41,42, and they may develop clinical disease following colonization 43. In turn, colonized health care workers can serve as vehicles for the transmission of S. aureus to patients. In fact, nosocomial outbreaks are frequently attributed to colonization of the nares and hands of health care workers 44,45.
Antimicrobial Resistance Trends
The propensity of S. aureus to develop resistance to virtually all the antimicrobial agents available to date has had a monumental impact on clinical infectious diseases. The present day epidemiology of staphylococcal infections has been shaped to a great extent by the rising antibiotic resistance rates commensurate with selective antibiotic pressure.
Resistance to beta-lactams
The first report of penicillinase-producing S. aureus was published in 1940, almost a year before penicillin was marketed for clinical use 46. Since then, beta-lactamase mediated penicillin resistance has been widely described among S. aureus isolates, with 80%-93% resistance rates currently reported in the hospital and the community 47-49.
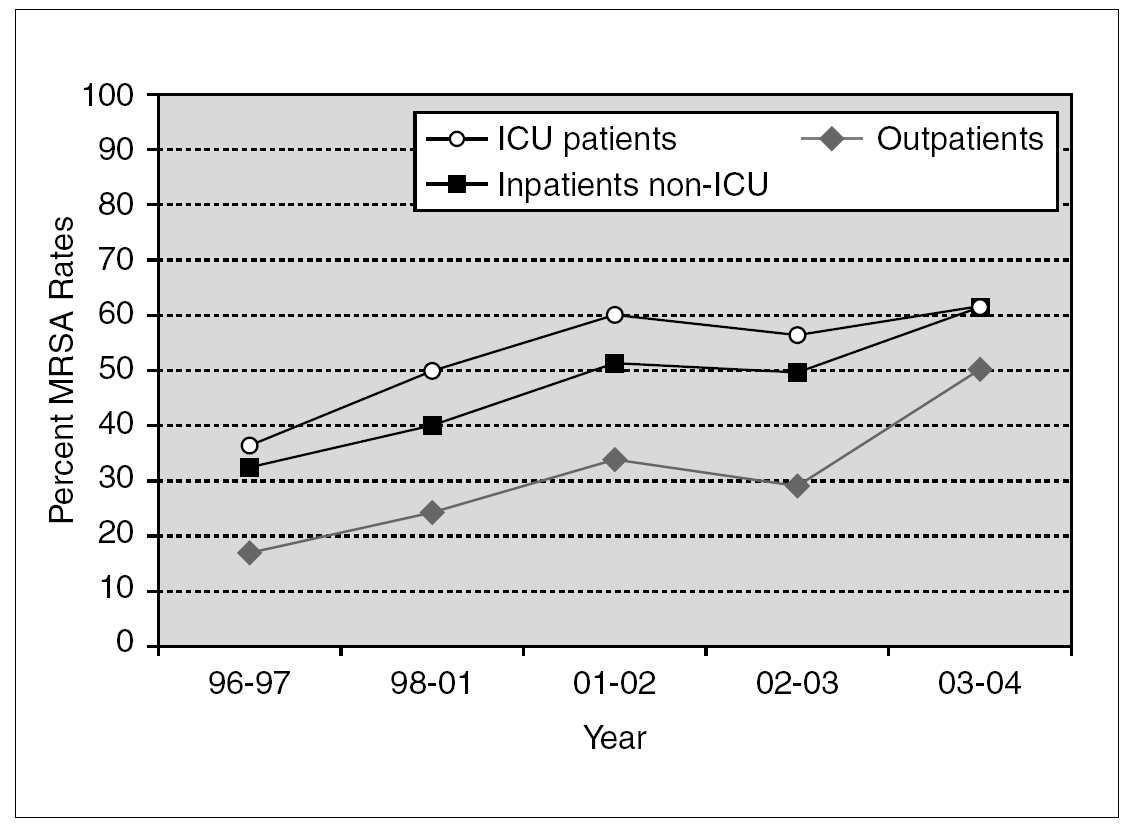
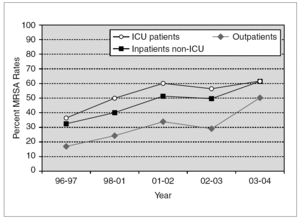
Penicillinase-stable cephalosporins and semisynthetic penicillins were introduced in the late 1950s. Once again, S. aureus was quick to develop resistance and MRSA isolates were described shortly thereafter 50. Methicillin resistance has been steadily increasing. According to data from the National Nosocomial Infections Surveillance (NNIS) System, the prevalence of MRSA among hospitalized patients rose from 31.9% in 1996 to 60.7% in 2004 (fig. 1) 51-55. Similar trends have been observed worldwide, although actual MRSA prevalence is subject to wide geographical variation. For instance, in Europe, MRSA rates as high as 58.0% in Italy and 54.0% in Portugal have been recently reported 56. In Japan, nearly 70% of S. aureus bloodstream isolates in 2001 were methicillin-resistant 57. On the other hand, Scandinavian countries have consistently noted very low rates of MRSA 58. Several risk factors have been independently associated with nosocomial MRSA colonization and infection, particularly in patients admitted to an intensive care unit (ICU). These include old age, severity of illness, length of ICU stay, multiple antibiotic use, mechanical ventilation, and the use of invasive medical devices (central venous catheters, urinary catheters, feeding tubes) 59.
Figure 1. Temporal trends of MRSA rates according to data from the NNIS System. MRSA: methicillin-resistant S. aureus; NNIS: National Nosocomial Infection Surveillance; ICU: intensive care unit.
Although initially confined to the hospital setting, MRSA isolates are now increasingly encountered in the community. Over the past decade, community-acquired MRSA (CA-MRSA) has quickly become a public health problem of epidemic proportions 60,61. NNIS data suggest that in 2004, 50.5% of S. aureus isolated from outpatients were methicillin-resistant (fig. 1) 53. In addition, a recent meta-analysis reported a 30.2% rate of community-onset MRSA infections from 27 studies. These figures, however, include outpatients with healthcare-associated infections. When applying strict definitions and excluding patients with healthcare-associated risk factors, CA-MRSA rates vary from 18.0 to 25.7 cases per 100,000 population 62. Multiple outbreaks of invasive infections caused by CA-MRSA have been described 63-65. Susceptible populations include children in day care centers 8, athletic teams 66, Native American communities 67, military personnel 68, and prison inmates 69. Patients with CA-MRSA commonly present with suppurative skin infections or necrotizing pneumonia. The ability of the organism to produce such invasive infections has been associated with Panton-Valentine leukocidin (PVL), a hemolysin encoded by a pvl gene located on a mobile phage that can be transmitted to other strains 70. The presence of pvl and other distinct bacterial genetic characteristics, including the presence of staphylococcal chromosomal cassette 4 (SCCmec 4) have been associated with severe cutaneous and pulmonary infections caused by community-acquired MRSA strains 71,72. Recent reports document that the epidemiology of CA-MRSA is increasingly blurring with that of hospital-acquired MRSA. A recent report from Atlanta documented that USA300, the most common CA-MRSA clone in the United States, is also a frequent cause of nosocomial and healthcare-associated bacteremia 73.
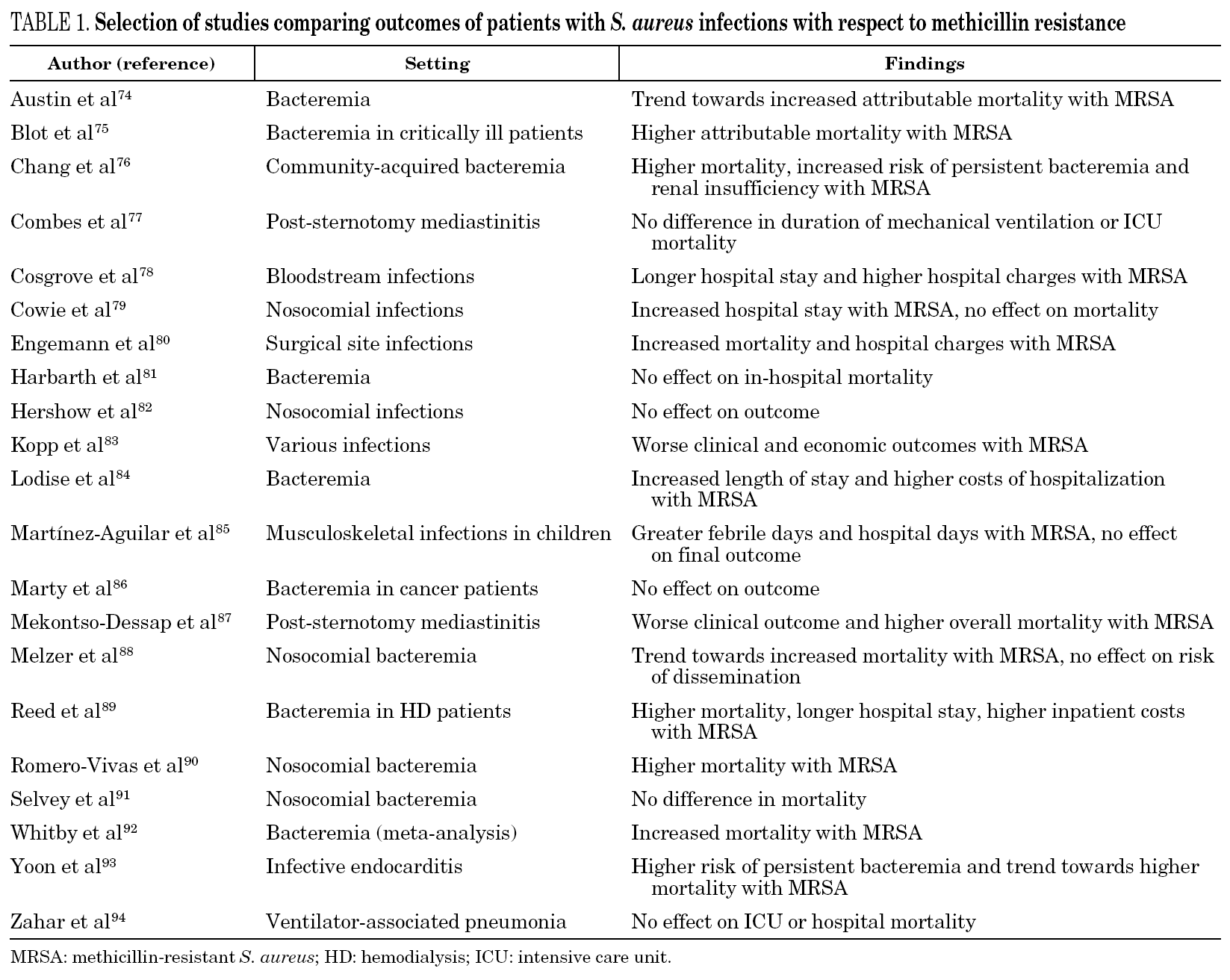
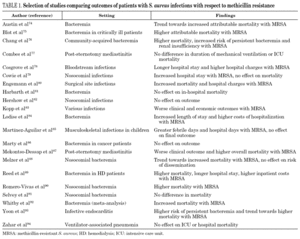
The effect of methicillin resistance on patient outcome has been a matter of intense debate. A number of studies addressing this issue have noted conflicting results in the setting of various S. aureus infections and various patient populations (table 1) 74-94. Whether the deleterious effect of MRSA observed in some of these studies is due to inherent virulence of the resistant strains or rather related to failure of vancomycin therapy remains unsettled. The advent of new antimicrobial agents with superior bactericidal activity compared to vancomycin will provide better chances in the future to accurately determine the independent effect of methicillin resistance through careful adjustment for the comorbid conditions of individual patients.
Resistance to glycopeptides
Staphylococcus aureus isolates with intermediate and high-level resistance to glycopeptides have been reported 95,96. Different mechanisms account for the two types of resistance. Vancomycin-intermediate S. aureus (VISA) harbor mutations that result in thickening of the peptidoglycan layer 97,98. Such resistance might be overcome with high doses of vancomycin. Conversely, vancomycin-resistant S. aureus (VRSA) have acquired the VanA resistance gene from enterococcal species and therefore do not exhibit a dose-dependent resistance to vancomycin 95,99. Although vancomycin resistance rates are still low, the emergence of such strains might be inevitable, especially with the continued pressure posed by intense glycopeptide use.
Diagnosis
Sites of staphylococcal infection are usually teeming with organisms. S. aureus grows on ordinary laboratory media and can be readily recognized on Gram stains from most clinical specimens 100. Definitive identification then relies on the tube or slide coagulase test 101,102, followed by antibiotic susceptibility testing through disk diffusion 103 or tube-dilution techniques 104. This method for MRSA identification relies on growing the organism in culture and then performing susceptibility testing; therefore it has a turnaround time of 48-72 hours. Recently developed polymerase chain reaction (PCR) assays provide a more rapid means for identifying MRSA isolates, and are especially valuable in detecting nasal colonization and bloodstream infections 105-107. Similar assays can now detect the pvl gene in clinical S. aureus isolates 108,109.
During outbreaks, phage typing of staphylococci is useful for recognizing the epidemic strain. More recently, molecular typing methods have provided reliable results. These include restriction endonuclease analysis of plasmid DNA 110, pulsed-field gel electrophoresis of DNA 111, and polymerase chain reaction amplification of selected DNA sequences 112.
The serological diagnosis of S. aureus bacteremia has been evaluated 113. Antibodies to a variety of staphylococcal antigens have been tested including peptidoglycan, teichoic acid, S. aureus ultrasonicate, whole S. aureus cells, alpha-toxin, lipase and capsular polysaccharide. Whole cell ELISA has been shown to be the most sensitive assay although all tests lacked specificity. Studies suggest that the presence of antibodies to S. aureus teichoic acid might indicate a chronic deep seated infection, including endocarditis, chronic osteomyelitis, and septic arthritis, whereas patients with uncomplicated bacteremia, acute osteomyelitis, cellulitis, and meningitis frequently have negative titers 114.
Clinical Syndromes
Virtually any organ system is prone to infection with S. aureus. This review does not present an exhaustive discussion of all the clinical manifestations of staphylococcal infections as these are reviewed elsewhere 115,116. We rather focus on systemic infections that have been associated with significant morbidity and mortality and that represent diagnostic and therapeutic challenges for clinical infectious disease specialists.
Bacteremia
Staphylococcus aureus bacteremia is now classified into three categories: hospital-acquired, health care-associated, and community-acquired SAB 117. Hospital-acquired and health-care associated infections exhibit similar epidemiological characteristics: both are related to comparable risk factors, such as intravascular devices and comorbid conditions. On the other hand, community-acquired SAB traditionally afflicts intravenous drug users and otherwise healthy patients with infections at various sites 118,119. In addition, hospital-acquired and health-care associated SAB result in significantly greater mortality rates when compared to community-acquired SAB (39%, 29%, and 16%, respectively) 117. All three SAB categories have increased considerably over the last decade 120. From 1980 to 1989, rates of SAB reported to the NNIS system increased by 283% in non-teaching hospitals and 176% in large teaching hospitals 121. By 1998, S. aureus had become the second most common bloodstream isolate, contributing to 16% of all hospital-acquired bacteremias 122. In Finland, Lyytikainen and colleagues documented a 55% increase in the incidence of SAB from 1995 to 2001, primarily in the elderly 123. Similarly, community-acquired SAB is being encountered more frequently, particularly with the increasing prevalence of pvl-bearing MRSA isolates in individuals without health-care contact 124-126.
Another notable trend in SAB has been the spread of antimicrobial resistance. MRSA rates have recently witnessed a prominent rise as a result of widespread antibiotic use and poor adherence to infection control precautions 127; approximately 30% of SAB isolates in the United States are now methicillin-resistant 122. Resistance is more apparent in hospital-acquired (61%) and health-care associated SAB (52%) than in community-acquired SAB (14%) (P = .001) 117.
Approximately one-third of patients with SAB develop one or more complications 118,128-131. Acute systemic complications typically manifest within 48 hours of diagnosis; these include septic shock, acute respiratory distress syndrome, and disseminated intravascular coagulation. On the other hand, metastatic complications of SAB may only become evident several weeks later. In one large retrospective study, common sites of metastatic disease were joints (36%), kidneys (29%), central nervous system (28%), skin (16%), intervertebral disk (15%), lungs (15%), liver/spleen (13%), bone (11%), and heart valves (8%). Importantly, more than one metastatic site of infection was present in half of the cases 118. Distant foci of infection in SAB develop preferentially in populations with certain predisposing conditions: 1) Underlying cardiac disease, such as native valvular abnormalities, congenital heart disease, and prior infective endocarditis 132-134; 2) Prosthetic implants, such as prosthetic valves 135, intracardiac devices 136, and orthopedic implants 137; 3) Community-acquired SAB, due in part to the typically prolonged disease course and duration of bacteremia prior to detection 138,139; 4) Old age 140 and comorbid conditions such as hemodialysis 141 and infection with the human immunodeficiency virus 142. The absence of the aforementioned risk factors, however, does not exclude the presence of metastatic disease.
Endocarditis
Infective endocarditis (IE) complicates the course of SAB in ~12% of cases 76,143. In a recent large cohort of patients, S. aureus was the most common cause of native valve endocarditis 144. Recent years have witnessed a rise in the rates of IE due to S. aureus145-148. S. aureus is now the leading cause of IE in many parts of the world 3. This trend is mostly attributed to the increasing prevalence of healthcare-associated S. aureus IE that has accompanied the growing use of interventional procedures, intravascular catheters, and implantable devices 148-150. For instance, Fernandez-Guerrero et al reported a 10-fold increase in the number of cases of hospital-acquired IE (most of which were due to S. aureus) from 1978 to 1992 compared to the number of cases occurring from 1960 to 1975 146. The increasing frequency of S. aureus IE can also be ascribed to better recognition of the disease through the widespread application of echocardiography in evaluating patients with SAB 4.
Endocarditis in patients with SAB frequently involves normal cardiac valves and is seldom accompanied by the physical stigmata of IE, rendering the diagnosis of the disease difficult 149,151. In fact, reliance solely upon physical examination findings is likely to result in underdiagnosis of S. aureus IE in a large number of cases 132,152. Because of the difficulty in clinically identifying S. aureus IE, the use of echocardiography has been advocated to evaluate patients with SAB. Despite its limited sensitivity in detecting vegetations (64%), transthoracic echocardiography (TTE) is a widely available, non-invasive screening modality in the setting of SAB 153. Conversely, transesophageal echocardiography (TEE) offers significant advantages over TTE, including higher sensitivity in identifying IE (90%) 154, improved identification of IE complications 155-157, and an enhanced ability to exclude IE in patients with native valves (negative predictive value 100%) 158,159. Whether TTE or TEE should be employed in the initial screening of the patient presenting with SAB remains a controversial issue 160-162. TEE is currently highly favored at our institution for the evaluation of most patients with SAB. The authors believe that TEE is likely to be cost-effective to guide duration of therapy in patients with intravascular catheter-associated SAB 163 or for patients at higher risk for IE or associated complications 161.
Despite early diagnosis and appropriate therapy, IE following SAB is often associated with devastating and life-threatening sequelae. The overall mortality of S. aureus IE ranges from 19% to 65% 118,131,148,149,152. Other complications include heart failure (20-50%) 147,149,152, paravalvular cardiac abscesses (30-40%) 164,165, neurological manifestations (30%) 166,167, and systemic embolization (40%) 168.
Pneumonia
Staphylococcus aureus is a significant etiologic agent in lower respiratory tract infections that has become increasingly more common in the hospital setting 169,170. According to the NNIS System, S. aureus was responsible for 20% of nosocomial pneumonias between 1992 and 1997 170. Furthermore, in the European Prevalence of Infection in Intensive Care (EPIC) Study, S. aureus was the predominant infective agent, accounting for 31% of microbiologically proven cases of ventilator-associated pneumonia 171. Whereas methicillin-susceptible S. aureus (MSSA) is typically encountered in early-onset hospital acquired pneumonia (< 5 days after admission), MRSA gains importance in late-onset hospital-acquired pneumonia and particularly in ventilator-associated pneumonia 22,172. Nosocomial pneumonia due to MRSA entails significant mortality with rates ranging from 38% to 55% 173,174. As with other S. aureus infections, whether methicillin resistance by itself contributes to the poor outcome is still a matter of debate 169,174.
In addition to its role as a nosocomially acquired pulmonary pathogen, S. aureus has recently established itself as an emergent threat in the community. Necrotizing pneumonia and sepsis caused by community-acquired MRSA strains carrying pvl genes are being increasingly recognized 72,175-179. Afflicted patients are typically healthy individuals without any healthcare contact. These infections are characterized by multifocal involvement of various organs, including lungs, brain, heart, liver, and kidneys. The pathological feature in the lungs is extensive hemorrhagic necrosis of the pulmonary parenchyma 72,175,176,178,179. The mean case fatality rate is noted to be as high as 35% 72,175,176,178,179. Mortality seems to be tightly linked to the presence of the pvl gene; in a study of S. aureus pneumonia, the mortality rate was 32% in cases with pvl-positive strains, as compared to 6% in those with pvl-negative strains 177.
Staphylococcus aureus pneumonia can present in several different forms, often in parallel with distinct pathophysiological mechanisms: 1) Lobar pneumonia usually occurs as a result of aspiration. Patients are acutely ill with high fevers and productive cough. In severe infections, empyema, abscess formation, cavitation and pneumatoceles may be present 180,181; 2) Diffuse interstitial pneumonia usually follows microaspiration and often develops in conjunction with, or following viral pneumonia 182; 3) Peripheral localized areas of pneumonia are noted with hematogenous seeding of the lungs from septic emboli secondary either to right-sided endocarditis or to soft tissue or joint infection. In this type of S. aureus pneumonia, pleuritic chest pain is a hallmark feature whereas cough and sputum production are less likely 183,184.
Novel therapies for MRSA
The use of beta-lactams in the treatment of S. aureus infections has been greatly handicapped by the increasing prevalence of MRSA strains. Although vancomycin, the traditional alternative antimicrobial agent, still maintains in-vitro activity against the majority of MRSA isolates, clinical cure rates in serious infections are disheartening. Treatment failure rates exceeding 40% have been recently quoted for SAB 185 and S. aureus pneumonia 186 treated with vancomycin. This has kindled great interest in developing new treatment options for MRSA.
Quinupristin/dalfopristin
Quinupristin and dalfopristin belong to the streptogramin class of antibiotics. When combined, these two agents are bactericidal and act in synergy on the 50S ribosomal subunit to inhibit protein synthesis. Quinupristin/dalfopristin is active in-vitro against both MSSA and MRSA 187. The drug is approved by the Food and Drug Administration (FDA) only for the treatment of complicated skin and skin structure infections (cSSSI) due to MSSA 188. However, data from a small controlled trial have suggested that quinupristin/dalfopristin is equivalent to vancomycin in the treatment of catheter-related bacteremia caused by S. aureus or coagulase-negative staphylococci (50% clinical and bacteriological responses in both groups) 189. Another study compared in a randomized design quinupristin/dalfopristin to vancomycin in the treatment of nosocomial pneumonia. Although both drugs were comparable in clinical efficacy (56% vs. 58%, respectively), the number of episodes of pneumonia caused by S. aureus was relatively small in both arms 190. Quinupristin/dalfopristin has also showed promising results in experimental rat and rabbit models of S. aureus endocarditis alone 191 or in combination with various antimicrobial agents such as beta-lactams 192, aminoglycosides 193, rifampin 194, and vancomycin 195. Limited Compassionate Use Registry data are available regarding the use of quinupristin/dalfopristin as a treatment option in patients with serious MRSA infections who are failing or are intolerant of traditional therapy 196. However, the cost, the requirement for administration by central catheter, and the side effect profile have all limited the use of this agent 197,198.
Linezolid
Linezolid is an oxazolidinone antimicrobial agent that binds reversibly to the bacterial 23S ribosome, thereby inhibiting protein synthesis. As a result of reversible inhibition, linezolid exhibits bacteriostatic activity against S. aureus. A major advantage offered by this new drug is an oral bioavailability of approximately 100% 199. Linezolid is indicated for the treatment of MRSA in the setting of cSSSI including diabetic foot infections without osteomyelitis. It has similar clinical efficacy as vancomycin in such infections but was statistically superior to vancomycin with regard to bacterial eradication in patients with confirmed MRSA at baseline 200. More recently, linezolid obtained FDA approval for the treatment of nosocomial pneumonia 201,202. According to a recent pooled analysis of randomized studies, linezolid was not inferior to vancomycin in the treatment of SAB (55% vs. 52%, respectively for overall cure rate) 203. The use of linezolid in MRSA endocarditis has had conflicting results. Although some reports described successful outcomes 204-206, there have been recent cases of clinical failure (one of which was fatal) with linezolid despite favorable in-vitro susceptibility results 207,208. Consequently, the authors do not recommend the use of linezolid in the setting of MRSA endocarditis regardless of the antimicrobial susceptibility of the isolate.
Daptomycin
Daptomycin is a cyclic lipopeptide with rapid bactericidal activity against MRSA. It exerts its action by inserting itself into the bacterial cell membrane. Subsequent events that lead to bacterial cell killing are not fully understood but are thought to involve dissipation of membrane potential. Daptomycin is FDA-approved for the treatment of cSSSI due to S. aureus including MRSA. In two distinct Phase III trials in patients with cSSSI, daptomycin resulted in similar success rates as its comparatorssemisynthetic penicillin or vancomycin (71.5% and 71.1%, respectively) 209. Despite lacking a formal indication, daptomycin is being used considerably in the setting of SAB and S. aureus endocarditis 210,211. Currently, phase III trials are being conducted to evaluate the efficacy of daptomycin in staphylococcal bloodstream infections. Daptomycin is not indicated in the treatment of pneumonia: the drug is inhibited by pulmonary surfactant 212 and proved to be inferior to ceftriaxone in a Phase III trial 213.
Tigecycline
Tigecycline is a newly introduced glycylcycline derivative with structural homology to tetracyclines. This drug offers broad-spectrum antimicrobial coverage including MRSA through binding to the 30S ribosomal subunit. Tigecycline has received FDA approval for the treatment of cSSSI and complicated intraabdominal infections 214. In addition, animal models have shown promising results with tigecycline compared to vancomycin in MRSA endocarditis 215.
Dalbavancin
Dalbavancin is a semisynthetic glycopeptide characterized by a long half-life (9-12 days) that allows once-weekly administration. It exerts its potent activity against MRSA via inhibition of cell wall synthesis. Dalbavancin has shown positive results in Phase III studies in cSSSI 216 and in a Phase II study in catheter-related bloodstream infections 217. It is currently awaiting FDA approval for these indications.
Telavancin
Telavancin is an experimental lipoglycopeptide molecule characterized by two mechanisms of action: inhibition of bacterial peptidoglycan synthesis; and alteration of bacterial cell membrane permeability and depolarization. Telavancin exhibits bactericidal in-vitro activity against S. aureus isolates including MSSA, MRSA and VISA isolates. In animal infection models, telavancin was efficacious in the treatment of various MRSA infections including soft tissue infections 218, pneumonia 219, and endocarditis 220. In Phase II clinical trials, telavancin was compared to standard therapy (semisynthetic penicillin or vancomycin) in patients with cSSSI 221. Data from this study showed that telavancin was equivalent to standard therapy both in clinical cure in the all treated population (79% vs. 80%) as well as in microbiological eradication in the MRSA subgroup (82% vs. 69%; P = .043). Phase III trials designed to demonstrate superiority over vancomycin are currently underway in patients with cSSSI, uncomplicated bacteremia, and hospital-acquired pneumonia.
Immunotherapy
Since microbial adherence is central to the initiation and metastatic spread of S. aureus, the MSCRAMM (microbial surface components recognizing adhesive matrix molecules) family of bacterial surface adhesin proteins represents an excellent target for the development of novel immunotherapies. Tefibazumab is a humanized IgG monoclonal antibody with high affinity to clumping factor A, an MSCRAMM protein common to virtually all S. aureus strains. It interferes with S. aureus adherence to extracellular matrix proteins in vitro and may enhance opsonophagocytosis of S. aureus by polymorphonuclear leukocytes 222. In an animal model of S. aureus IE, addition of tefibazumab to vancomycin significantly increased bacterial clearance from the bloodstream when compared to vancomycin alone (P < .008) 223. The results of a Phase II randomized, double-blind, multi-center clinical study of tefibazumab in patients with SAB were recently presented 224.
Prevention
Nasal decolonization
Since MRSA nasal colonization frequently precedes infection, endeavors to contain the transmission of MRSA have targeted the eradication of nasal carriage in susceptible patients. Studies evaluating this strategy have yielded conflicting results. Cardiothoracic surgery patients who received mupirocin prophylaxis had a lower surgical wound infection rate than historical controls (7.3% vs. 2.8%; P < .001) 225. More recently, combining results from two randomized trials in surgical patients suggested that the administration of mupirocin in surgical patients reduced postoperative nosocomial S. aureus infections as compared to placebo (RR 0.49, 95% CI 0.29-0.83; number needed to treat 26) 226,227. Boelaert et al found a four- to six-fold reduction in SAB rates in hemodialysis patients receiving mupirocin 228. On the other hand, one study in nonsurgical patients failed to show a benefit from mupirocin prophylaxis with respect to rates of nosocomial S. aureus infections, in-hospital mortality, and duration of hospitalization 229. Investigators have therefore suggested that a single course of mupirocin may be insufficient in low-risk patients with prolonged exposure 230. In addition to conflicting messages from clinical trials, the emergence of mupirocin-resistance has also been reported 231,232.
Vaccination
Staphylococcus aureus Polysaccharide Conjugate Vaccine (StaphVax®, Nabi Biopharmaceuticals, Rockville, MD) is an investigational polysaccharide conjugate vaccine that presents a novel approach to the prevention of S. aureus infections. It consists of type 5 and type 8 capsular polysaccharides, the strains accounting for more than 80% of infections. In one double blinded, placebo-controlled Phase III clinical efficacy trial involving 1804 hemodialysis-dependent patients, StaphVax recipients failed to meet the a priori endpoint of reduction in episodes of S. aureus bacteremia at 54 weeks. However, post hoc analysis revealed a 57% reduction in SAB episodes at 10 months compared to placebo recipients (P = 0.015) 233. Based on these findings, a second Phase III confirmatory trial, with modified time points, was undertaken. However, this second trial also failed to meet its primary endpoint. As a result, all clinical trial development and further marketing of StaphVax have been held until assessment of the results is completed.
Infection control strategies
Several studies have established that the transmission of MRSA between patients within the hospital setting occurs to a great extent through health care workers 44,45. Consequently, the Centers for Disease Control and Prevention (CDC) recommend the implementation of contact precautions in patients colonized or infected with MRSA 234. Such precautions include the use of private rooms, protective attire for health care workers, and strict adherence to hand hygiene principles. There is abundant evidence to support the efficacy of these infection control programs in reducing the transmission of resistant pathogens within the hospital 20,235-240. Although active surveillance for MRSA and preemptive isolation of colonized or infected patients remains an integral part of many hospital infection control programs, observance of infection control guidelines has been suboptimal 241-243. Hand hygiene practices have been particularly inadequate 244-247. Accordingly, continuous efforts should be made to improve compliance with isolation and hand hygiene policies to prevent the dire consequences of nosocomial MRSA transmission.
Correspondence: Vance G. Fowler Jr., MD.
Division of Infectious Diseases.
Duke University Medical Center.
DUMC Box 3281.
Durham, NC 27710.
E-mail: fowle003@mc.duke.edu