Symkevi®(tezacaftor-ivacaftor) is indicated in a combination regimen of 150mg ivacaftor and 100mg tezacaftor pills for the treatment of patients with cystic fibrosis (CF) 6 years of age or older who are homozygous for the F508del mutation or heterozygous for the F508del mutation with residual function.1 Tezacaftor is a selective corrector of the altered or deficient CF protein, cystic fibrosis transmembrane conductance regulator (CFTR), which facilitates cellular processing and transport of CFTR to the cell surface. Ivacaftor is a CFTR protein enhancer that increases the opening of the CFTR channel at the cell surface. The combination works against the abnormal CFTR protein, increasing the amount and function of CFTR at the cell surface, resulting in an increase in airway surface fluid volume and ciliary beating frequency in vitro in human bronchial epithelial cells.2 This drug is available in Spain from 1st October 2019.3 The various clinical trials conducted with this drug demonstrate its clinical efficacy on lung function, reduction of chloride concentration in sweat, improvement of body mass index, quality of life, as well as a 35% reduction in the number of pulmonary exacerbations compared to the placebo group.4,5 To date, there are no published data on the tezacaftor-ivacaftor effect on microbiological cultures.
The aim of this study is to assess, in real life, the effects on sputum microbiological cultures in adult CF patients from different units in Spain who received the combination tezacaftor-ivacaftor for 1 year.
We conducted an ambispective, multicentre study from December-1st 2019 to December-31st 2021 in the following CF Units in Spain: Hospital Universitario Virgen del Rocío (Seville), Hospital Universitario La Princesa (Madrid), Hospital Universitario 12 de Octubre (Madrid), Hospital Universitario La Paz (Madrid), Hospital Universitario Cruces (Bilbao), Hospital Universitario Central de Asturias, Hospital Carlos Haya (Málaga) and Hospital Universitario de la Coruña. Patients 18 years of age or older, who met diagnostic criteria for CF6 and who received a consecutive dose of 100mg tezacaftor/150mg ivacaftor in the morning+150mg ivacaftor in the evening (12-h interval) were included. Microbiological cultures were recorded every 6 months for 1 year and compared with the 6 and 12 months prior to taking the drug. The percentage of change was calculated comparing the differences in bacteria present at 12 month pre-treatment with 12 month post-treatment. Finally, the comparison of the proportions for each bacterial species at 12 and 6 months pre-treatment versus 6 and 12 months post-treatment was assessed using a χ2 test or Fisher's exact test, whenever required. All analyses were performed using R statistical software.
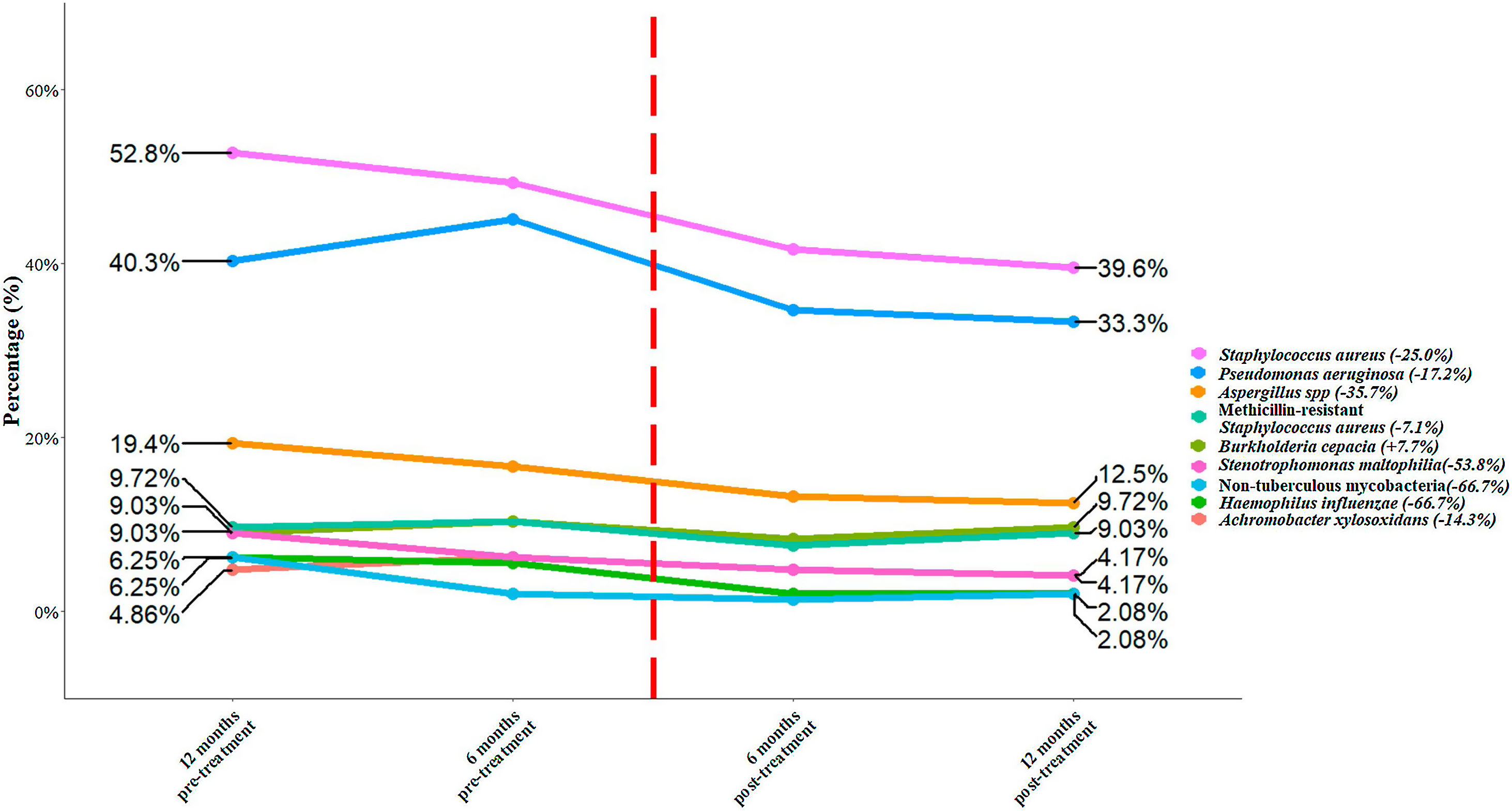
We included a total of 144 patients with an age of 31.2 (± 9.5) years. Among the total of patients, 78 (54.2%) were male, 111 (77.1%) were homozygous F508del and 33 (22.9%) heterozygous F508del. There were 120 (83.3%) patients with pancreatic insufficiency and 43 (29.9%) with CF-related diabetes. Chronic bronchial infection was present in 84.0% of the patients. The most prevalent microorganisms were Staphylococcus aureus (52.8%), Pseudomonas aeruginosa (40.3%), and methicillin-resistant S. aureus (MRSA) (9.7%). The percentage of change between 12 months pre-treatment and post-treatment showed a reduction for all bacteria species, except for Burkholderia cepacia (Fig. 1). The top five bacteria with the highest percentages of reduction were non-tuberculous mycobacteria (−66.7%), Haemophilus influenzae (−66.7%), Stenotrophomonas maltophilia (−53.8%), Aspergillus spp. (−35.7%) and S. aureus (−25.0%). Finally, we assessed whether the administration of the treatment significantly reduced the presence of any bacterial species. S. aureus showed a significant reduction (p=0.033) between 12 month pre-treatment to 12 post-treatment, while the remaining bacterial species did not show a significant reduction (p≥0.054).
Percentage of bacteria at 12- and 6-months pre-treatment and at 6 and 12 months post-treatment. The flashing red line symbolizes time 0 of the application of the treatment. The percentages of change for each bacterial species between 12 months pre-treatment and 12 months post-treatment is shown in brackets.
Currently, the publications showing the results obtained after the use of this modulatory drug in daily clinical practice in CF units are scarce.7 In CF, respiratory tract involvement remains the main cause of morbidity and mortality.1 Chronic bronchial infection in CF, especially by P. aeruginosa, MRSA or B. cepacia causes airway inflammation, triggers pulmonary exacerbations, reduces quality of life and is an independent risk factor for increased mortality. Therefore, the trend towards a reduction of microorganisms in respiratory samples is a relevant factor in the evolution of the disease. The reduction of P.aeruginosa has been described in several studies in patients with ivacaftor treatment with gating mutations (non-functional surface CFTR protein).8,9 Only a small study of 20 homozygous F508del patients with another modulator combination, lumacaftor-ivacaftor, showed a non-significant decrease in P. aeruginosa.10
We believe that the present study is of great importance because it is the first multicentre experience presenting microbiological data with the combination of tezacaftor-ivacaftor modulators in a population of Spanish adults with CF.
Beatriz Gómez Crespo (Hospital Universitario de Cruces, Bilbao, Spain)
Layla Diab Cáceres (Hospital Universitario 12 de octubre, Madrid, Spain)
Mª Teresa Tejedor Ortiz (Hospital Universitario 12 de octubre, Madrid, Spain)
Marta García Clemente (Hospital Universitario Central de Asturias, Spain)
Marta Solís García (Hospital Universitario de la Princesa, Madrid, Spain, Facultad de Medicina, Universidad Autónoma de Madrid, Madrid, Spain)
Lucia González Torres (Hospital Universitario A Coruña, Spain)
Marina Blanco Aparicio (Hospital Universitario A Coruña, Spain)
Casilda Olveira (Hospital Regional Universitario de Málaga, Málaga, Spain)
M.ª Victoria Girón-Fernández (Hospital Regional Universitario de Málaga, Spain)
Ester Zamarrón de Lucas (Hospital Universitario La Paz, Madrid, Spain)
Concha Prados Sanchez (Hospital Universitario La Paz, Madrid, Spain)
Esther Quintana Gallego (Hospital Universitario Virgen del Rocío. Sevilla, Spain)
Teresa Alarcón (Hospital Universitario de la Princesa, Madrid, Spain)
María del Carmen Ruiz Gallego (Hospital Universitario Virgen del Rocío, Sevilla, Spain)
Elena Urra Zalbidegotia (Hospital Universitario de Cruces, Bilbao, Spain)
M.a Ángeles Orellana (Hospital Universitario 12 de octubre, Madrid, Spain)
Javier Fernández Dominguez (Hospital Universitario Central de Asturias, Spain)
M.ª Begoña Fernández Pérez (Hospital Universitario A Coruña, Spain)
M.ª Pilar Bermúdez Ruiz (Hospital Regional Universitario de Málaga, Málaga, Spain)
Julio García Rodríguez (Hospital Universitario La Paz, Madrid, Spain)