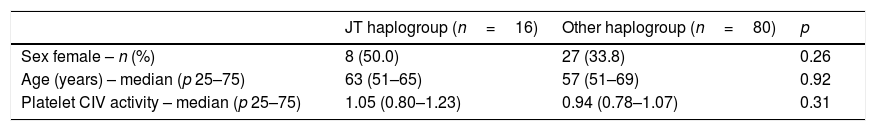The comparison on mitochondrial function between severe septic patients and healthy control subjects according to mitochondrial deoxyribonucleic acid (mtDNA) haplogroup has not been previously reported; and this was the objective of the current study.
MethodsProspective, multicenter, observational study. We obtained blood samples from 198 severe septic patients at days 1, 4 and 8 of severe sepsis diagnosis and from 96 sex- and age-matched healthy controls to determine mtDNA haplogroup and platelet respiratory complex IV (CIV) specific activity. The endpoint of the study was 30-day mortality.
ResultsWe included 198 severe septic patients (38 with mtDNA haplogroup JT and 160 with mtDNA haplogroup non-JT) and 96 healthy control subjects (16 with mtDNA haplogroup JT and 80 with mtDNA haplogroup non-JT). We have no found statistically significant differences in platelet CIV specific activity between healthy controls and survivor severe septic patients with mtDNA haplogroup JT at days 1, 4 and 8 of severe sepsis diagnosis; and the remaining severe septic patients showed lower platelet CIV specific activity than healthy controls with the same mtDNA haplogroup.
ConclusionsThe new finding of our study was that survivor severe septic patients and healthy controls with mtDNA haplogroup JT showed no different platelet Civ specific activity.
La comparación en la función mitocondrial entre pacientes con sepsis grave y sujetos sanos según el haplogrupo del ácido desoxirribonucleico mitocondrial (ADNmt) no se ha reportado previamente; y este fue el objetivo del estudio.
MétodosEstudio prospectivo, multicéntrico y observacional. Obtuvimos muestras sanguíneas de 198 pacientes con sepsis grave en los días 1, 4 y 8 del diagnóstico de la sepsis grave y de 96 sujetos sanos para determinar el haplogrupo del ADNmt y la actividad del complejo respiratorio mitocondrial IV (CIV) en plaquetas circulantes. La variable resultado principal del estudio fue la mortalidad a los 30 días.
ResultadosSe incluyeron 198 pacientes con sepsis grave (38 con haplogrupo JT del ADNmt y 160 con otro haplogrupo del ADNmt) y 96 sujetos sanos (16 con haplogrupo JT del ADNmt y 80 con otro haplogrupo del ADNmt). No encontramos diferencias estadísticamente significativas en la actividad de CIV plaquetaria entre los sujetos sanos y los pacientes sépticos supervivientes con haplogrupo JT del ADNmt en los días 1, 4 y 8 del diagnóstico de la sepsis grave; y el resto de los pacientes sépticos presentaron menor actividad de CIV plaquetaria que los sujetos sanos con su mismo haplogrupo del ADNmt.
ConclusionesEl nuevo hallazgo de nuestro estudio fue que los pacientes sépticos y sujetos sanos con haplogrupo JT del ADNmt no tenían diferencias en la actividad de CIV plaquetaria.
Sepsis is associated with considerable resource consumption, and mortality.1,2 Findings as the presence of elevated tissue oxygen concentrations in skeletal muscle of septic patients3,4 and that non-survivor septic patients did not response with an increase in tissue oxygen consumption following to the increase of oxygen delivery5 rise the theory that during sepsis there is a mitochondrial dysfunction leading to a cellular inability to use oxygen.6 Most of oxygen delivered to tissues is consumed by the mitochondrial respiratory chain of the oxidative phosphorylation (OXPHOS) system to produce adenosine triphosphate (ATP), and the respiratory complex IV (CIV) or cytochrome c oxidase is the highest responsible of this oxygen consumption.
The mitochondrial dysfunction has been scarcely analyzed in patients with sepsis, and the most of studies with small sample size.7–27 In addition, the influence of mitochondrial deoxyribonucleic acid (mtDNA) haplogroup on survival of septic patients has been scarcely studied.28–32 Previously, our team found that non-surviving septic patients showed lower platelet respiratory complex IV (CIV) specific activity than survivors at the time of severe sepsis diagnosis25 and during the first week of severe sepsis,26 and that septic patients showed lower platelet CIV specific activity than healthy controls.27 In addition, our team found that patients with mtDNA haplogroup JT compared with other haplogropus showed higher survival at 1 month30 and 6 months,31 and higher platelet CIV specific activity.32 However, the comparison on mitochondrial function between severe septic patients and healthy control subjects according to mtDNA haplogroup has been not previously reported; and this was the objective of the current study.
Material and methodsDesign and subjectsThis prospective, multicenter, and observational study was carried our in six Intensive Care Units (ICUs) from Spain. The study was approved by the Institutional Ethic Review Boards of all hospitals: San Jorge (Huesca), Clínico Universitario de Valencia (Valencia), Universitario de Canarias (La Laguna. Tenerife), Insular (Las Palmas de Gran Canaria), Universitario Dr. Negrín (Las Palmas de Gran Canaria), Universitario Nuestra Señora de Candelaria (Santa Cruz de Tenerife). Patients or family members signed the informed consent to participate in the study.
We included 198 patients with severe sepsis diagnosed according to the International Sepsis Definitions Conference criteria.33 We excluded patients with white blood cell count <1000cells/μl, human immunodeficiency virus (HIV), solid or hematological tumor, steroid or immunosuppressive or radiation therapy, age <18 years, lactation, pregnancy and platelet transfusion. We also included 96 sex- and age-matched healthy subject controls.
The same cohort of severe septic patients was used in previous publications by our team to compare platelet CIV specific activity between surviving and non-surviving severe septic patients and between septic patients and healthy controls, and to compare severe sepsis survival and platelet CIV specific activity according to mtDNA.25,30–32 In the current analysis, we compare mitochondrial function between severe septic patients and healthy control subjects according to mtDNA haplogroup due to it has not been previously reported.
Determination of mtDNA haplogroup and platelet CIV specific activityWe recollected blood samples from healthy controls and from severe septic patients at days 1, 4 and 8 of the diagnois of severe sepsis, and then were frozen at −80°C. The determination of mtDNA haplogroup and platelet CIV specific activity was centralized in the Departamento de Bioquímica y Biología Molecular y Celular of the Centro de Investigaciones Biomédicas En Red de Enfermedades Raras (CIBERER) e Instituto de Investigación Sanitaria de Aragón (Universidad de Zaragoza. Zaragoza. Spain).
For the determination of mtDNA haplogroup determination was used real time-polymerase chain reaction. In all blood samples were genotyped three single nucleotide polymorphisms (m.4216C, m.7028C and m.12308G) that define very frequent haplogroups (JT, H and U). We analyzed other 14 mtDNA single nucleotide polymorphisms (m.15693C, m.15257A, m.15218G, m.14798C, m.14793G, m.14766C, m.13708A, m.10873C, m.9477A, m.4769A, m.4580A, m.4336C, m.3010A, m.1811G) by an hierarchic approach to determine each particular haplogroup.34 We considered patients with mtDNA haplogroup non-JT to those patients with a mtDNA haplogroup different to JT.
In respect to platelet CIV specific activity, first we obtained platelets according to a protocol previously described.35 We determined protein levels using a protocol previously described,36 and CIV activity by means of Mitoprofile Human Complex IV Activity kit (Mitosciences, Invitrogen, Carlsbad, CA). CIV specific activity was expressed as milli-optic density/min/mg multiplied by 100.
Statistical analysisWe reported categorical variables in form of frequencies and percentages, and continuous variables in form of medians and interquartile ranges. We compared categorical variables between groups using chi-square test, and continuous variables by Wilcoxon–Mann–Whitney test. All P values lower than 0.05 were considered statistically significant; and Bonferroni correction was used to correct multiple comparisons in repeated measures. The statistical analyses were performed with SPSS v. 17.0 (SPSS Inc., Chicago, IL, USA) and Med Calc v. 10.1.3.0 (Mariakerke, Belgium) programs.
ResultsWe included 198 severe septic patients, 38 with mtDNA haplogroup JT and 160 with mtDNA haplogroup non-JT (80 HV, 51 U and 29 Non-R). We also included 96 healthy control subjects, 16 with DNAmt haplogroup JT and 80 with mtDNA haplogroup non-JT (26 HV, 42 U and 12 Non-R).
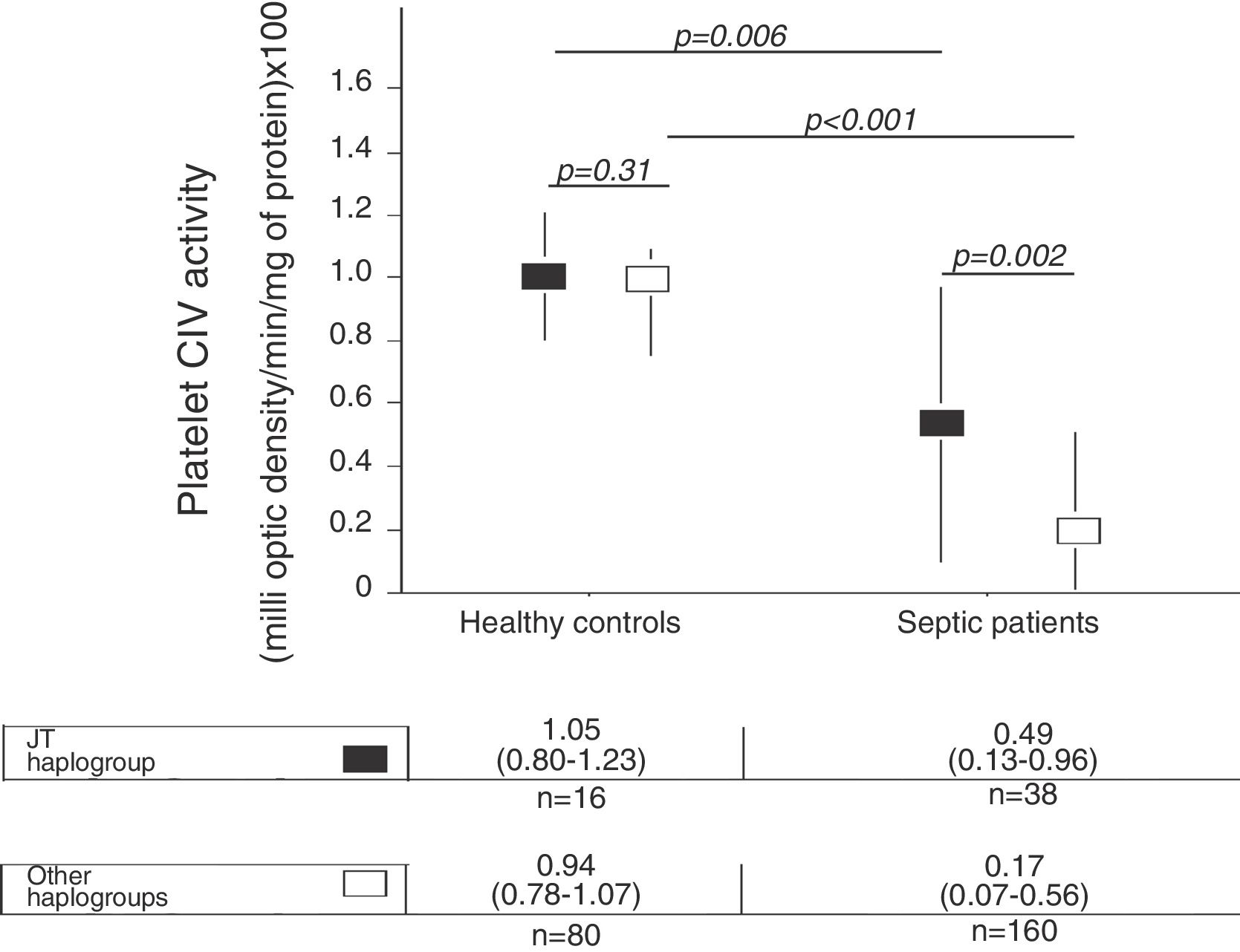
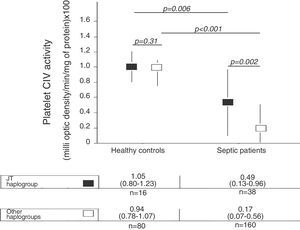
There were not statistically significant differences in age, sex and platelet CIV specific activity between healthy control subjects with mtDNA haplogroup JT and other mtDNA haplogroups (Table 1, Fig. 1).
Demografic variables in healthy controls subjects according to DNAmt haplogroup.
| JT haplogroup (n=16) | Other haplogroup (n=80) | p | |
|---|---|---|---|
| Sex female – n (%) | 8 (50.0) | 27 (33.8) | 0.26 |
| Age (years) – median (p 25–75) | 63 (51–65) | 57 (51–69) | 0.92 |
| Platelet CIV activity – median (p 25–75) | 1.05 (0.80–1.23) | 0.94 (0.78–1.07) | 0.31 |
Platelet respiratory complex IV (CIV) specific activity is expressed as milli optical density/min/mg of protein.
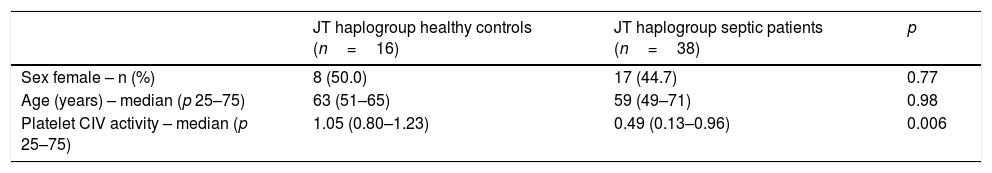
We no found statistically significant differences in age and sex between healthy control subjects and severe septic patients with mtDNA haplogroup JT; however, severe septic patients showed lower platelet CIV specific activity (p=0.006) than healthy control subjects (Table 2, Fig. 1).
Demografic variables in healthy controls and severe septic patients with mtDNA haplogroup JT.
| JT haplogroup healthy controls (n=16) | JT haplogroup septic patients (n=38) | p | |
|---|---|---|---|
| Sex female – n (%) | 8 (50.0) | 17 (44.7) | 0.77 |
| Age (years) – median (p 25–75) | 63 (51–65) | 59 (49–71) | 0.98 |
| Platelet CIV activity – median (p 25–75) | 1.05 (0.80–1.23) | 0.49 (0.13–0.96) | 0.006 |
Platelet respiratory complex IV (CIV) specific activity is expressed as milli optical density/min/mg of protein.
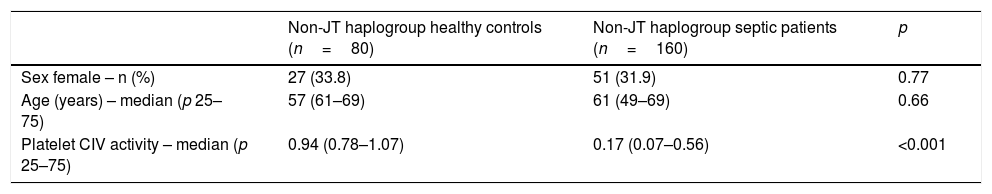
There were not statistically significant differences in age and sex between healthy control subjects and severe septic patients with mtDNA haplogroup non-JT; however, severe septic patients showed lower platelet CIV specific activity (p<0.001) than healthy control subjects (Table 3, Fig. 1).
Demografic variables in healthy controls and severe septic patients with mtDNA haplogroup non-JT.
| Non-JT haplogroup healthy controls (n=80) | Non-JT haplogroup septic patients (n=160) | p | |
|---|---|---|---|
| Sex female – n (%) | 27 (33.8) | 51 (31.9) | 0.77 |
| Age (years) – median (p 25–75) | 57 (61–69) | 61 (49–69) | 0.66 |
| Platelet CIV activity – median (p 25–75) | 0.94 (0.78–1.07) | 0.17 (0.07–0.56) | <0.001 |
Platelet respiratory complex IV (CIV) specific activity is expressed as milli optical density/min/mg of protein.
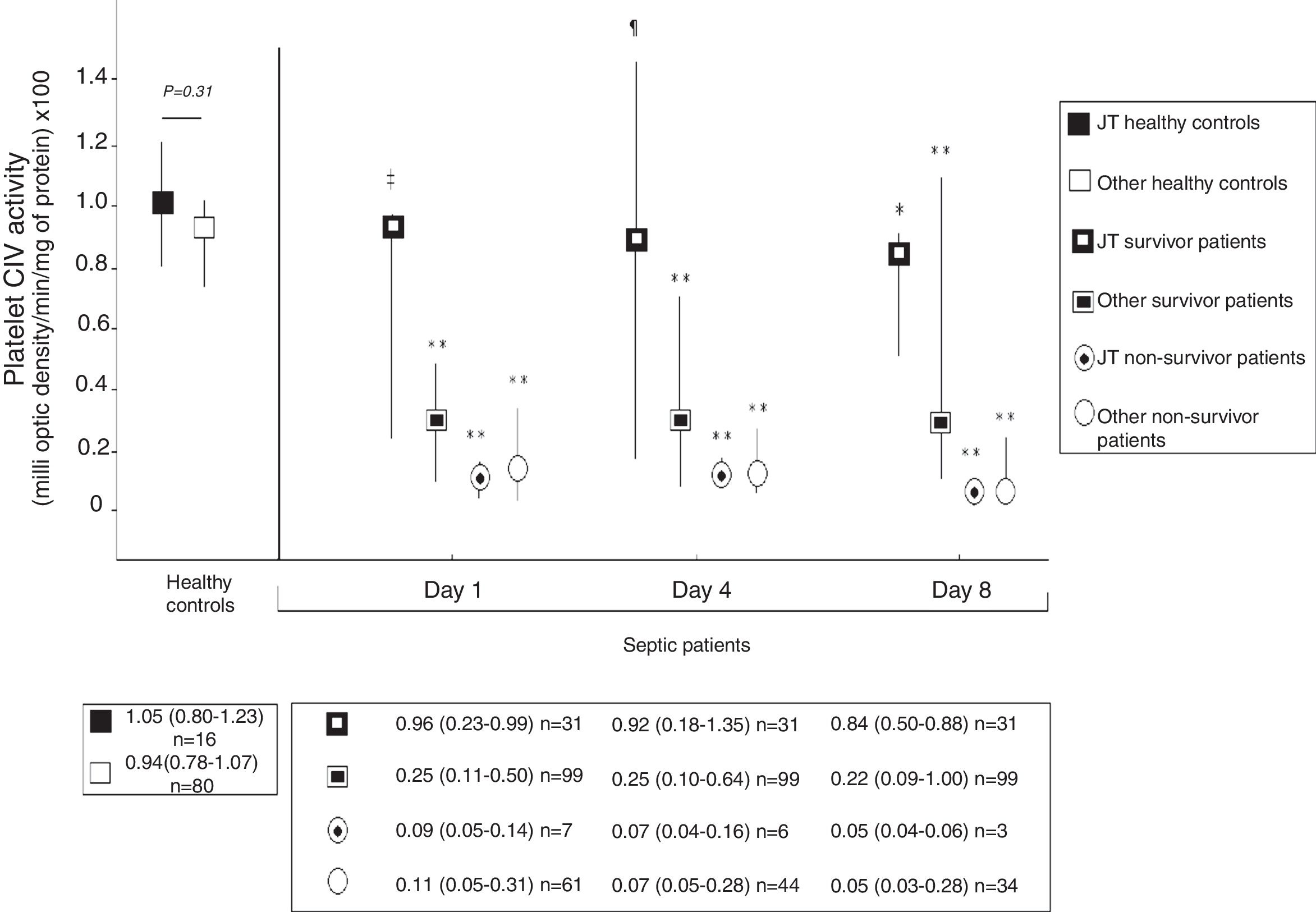
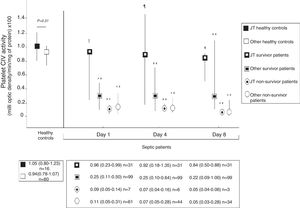
Fig. 2 shows platelet CIV specific activity in healthy controls, and survivor and non-survivor severe septic patients during follow up according to mtDNA haplogroup. We found that healthy control subjects with mtDNA haplogroup non-JT showed higher platelet CIV specific activity than survivor and non-survivor severe septic patients with mtDNA haplogroup non-JT at days 1, 4 and 8 of severe sepsis diagnosis. We found that healthy control subjects with mtDNA haplogroup JT showed higher platelet CIV specific activity than non-survivor severe septic patients with mtDNA haplogroup JT at days 1, 4 and 8 of severe sepsis diagnosis; however, we have no found statistically significant differences in platelet CIV specific activity between healthy control subjects and survivor severe septic patients with mtDNA haplogroup JT at days 1, 4 and 8 of severe sepsis diagnosis.
Platelet respiratory complex IV (CIV) specific activity in healthy controls, and survivor and non-survivor severe septic patients during follow up according to mtDNA haplogroup. The p-values for the comparisons on platelet CIV specific activities between healthy controls and severe septic patients were the following: ‡p=0.04, **p<0.001, ¶p=0.15, *p=0.01. All p-values lower than 0.004 were statistically significant after Bonferroni correction.
To our knowledge, this is the first study reporting the comparison on mitochondrial function between severe septic patients and healthy control subjects according to mtDNA haplogroup. The novel findings of our study were the following: 1) Healthy control subjects with mtDNA haplogroup non-JT showed higher platelet CIV specific activity than survivors and non-survivors severe septic patients with mtDNA haplogroup non-JT during the first week of severe sepsis diagnosis; 2) Healthy control subjects with mtDNA haplogroup JT showed higher platelet CIV specific activity than non-survivors severe septic patients with mtDNA haplogroup JT during the first week of severe sepsis diagnosis; 3) There were not found differences in platelet CIV specific activity between healthy control subjects and survivor severe septic patients with mtDNA haplogroup JT during the first week of severe sepsis diagnosis.
Previously, there was reported a lower OXPHOS function in septic patients compared to healthy control subjects in muscle,7–11 in peripheral blood mononuclear cells13,15–17 and in circulating platelets.20,21,23,24,27 In addition, there was found a lower OXPHOS function in circulating platelets in non-survivor than in survivor septic patients.21,25,26 Besides, there was found an association between mtDNA haplogroup and survival rate in septic patients,28–32 and an association between mtDNA haplogroup and platelet CIV specific activity.32 Thus, the more important and new finding of our study was that survivor severe septic patients with mtDNA haplogroup jt showed no different platelet Civ specific activity than healthy control subjects with mtDNA haplogroup jt; and that the remaining severe septic patients showed lower platelet CIV specific activity than healthy control subjects with the same mtDNA haplogroup. Then, it is possible that in some patients the mtDNA haplogroup jt could helps them to maintain the OXPHOS function and survive to severe sepsis; however, in other patients with mtDNA haplogroup jt, the apparently beneficial mtDNA haplogroup cannot compensate the sepsis process and the patients do not survive to sepsis. Finally, we think that is biologically plausible that patients with mtDNA haplogroup JT (polymorphism) have lower mitochondrial dysfunction during sepsis (intermediate phenotype) and lower risk of death during sepsis (clinical phenotype).
We think that the findings of our study could have clinical implication in respect to prognosis and treatment of septic patients. We think that the findings of our study could suggest that the determination of the mtDNA haplogroup could help in the estimation of septic patients who have more risk of death, could increase the interest for research on how improve mitochondrial function in septic patients with unfavorable mtDNA haplogroup and could help in the selection of patients who may benefit of agents used with that intention.
Our study showed some limitations. First, there were not analyzed ATP synthesis, other mitochondrial respiratory complexes, mixed venous oxygen saturation (SvO2), mitochondrial inner membrane potential, or reactive oxygen species (ROS) to explore the function of OXPHOS system. Second, there was not analyzed mitochondrial function in other cells. Third, as the study was developed before to the new criteria of sepsis,37 we used the previous sepsis criteria.33
ConclusionsTo our knowledge, this is the first study reporting the comparison on mitochondrial function between severe septic patients and healthy control subjects according to mtDNA haplogroup. The more important and new finding of our study was that survivor severe septic patients and healthy control subjects with mtDNA haplogroup jt showed no different platelet CIV specific activity; and that the remaining severe septic patients showed lower platelet CIV specific activity than healthy control subjects with the same mtDNA haplogroup.
FundingThis study was supported by grants from Instituto de Salud Carlos III (PI-14-00005, PI-14-00070, and INT16/00165) (Madrid, Spain) and co-financed with Fondo Europeo de Desarrollo Regional (FEDER).
Conflict of interestsThe authors declare that they have no competing interests.