Factors predicting short-term amputation during hospital treatment of patients admitted for acute diabetic foot infections are of interest for clinicians managing the acute episode.
MethodsA retrospective clinical records analysis of 78 consecutive patients hospitalized for acute diabetic foot infections was performed to identify predictive factors for short-term amputation by comparing the data of patients who ultimately required amputation and those who did not. Clinical/epidemiological, laboratory, imaging, and treatment variables were comparatively analyzed. A logistic regression model was performed, with amputation as the dependent variable and factors showing significant differences in the bivariate analysis as independent variables. A prediction score was calculated (and validated by ROC curve analysis) using beta coefficients for significant variables in the regression analysis to predict amputation.
ResultsOf the 78 patients (70.5% with peripheral vasculopathy) included, 26 ultimately required amputation. In the bivariate analysis, white blood cell count, previous homolateral lesions, odor, lesion depth, sedimentation rate, Wagner ulcer grade, and arterial obstruction on Doppler study were significantly higher in patients ending in amputation. In the multivariate analysis, the risk of amputation was increased only by Wagner grade 4 or 5 (20-fold higher), obstruction (12.5-fold higher), and elevated sedimentation rate (6% higher per unit). Logistic regression predicted outcome in 76.9% of patients who underwent amputation and 92.3% of those who did not.
ConclusionThe score calculated using beta coefficients for significant variables in the regression model (Wagner grades 4 and 5, obstruction on Doppler, and elevated sedimentation rate for the clinical, imaging, and laboratory data, respectively) correctly predicted amputation during hospital management of acute diabetic foot infections.
La identificación de factores que predigan amputación temprana durante la hospitalización de pacientes ingresados por infección aguda de pie diabético es de alto interés para el clínico que trata el episodio agudo.
MétodosSe realizó un análisis retrospectivo de las historias clínicas de 78 pacientes consecutivos ingresados por infección aguda de pie diabético para identificar los factores que predicen amputación temprana mediante la comparación de los datos de los pacientes finalmente amputados con los de aquellos en los que finalmente no se realizó amputación. Se analizaron datos clínicos, epidemiológicos, analíticos, de imagen y de tratamiento. Se realizó un modelo de regresión logística utilizando como variable dependiente la amputación y como variables independientes aquellas que mostraron diferencias significativas en el análisis bivariado. Se calculó una escala de puntuaciones (validada por curvas de especificidad y sensibilidad) utilizando los coeficientes beta de las variables significativas en el modelo de regresión para predecir la amputación.
ResultadosDe los 78 pacientes incluídos (70,5% con vasculopatía periférica), 26 fueron finalmente amputados. En el análisis bivariado, el recuento de leucocitos, las lesiones homolaterales previas, el olor, la extensión de la lesión, la velocidad de sedimentación, el grado en la escala de Wagner y la obstrucción arterial en el Doppler fueron significativamente mayores en los pacientes finalmente amputados. En el análisis multivariado el riesgo de amputación solo se incrementó por los grados 4–5 de la escala de Wagner (20 veces), obstrucción en el Doppler (12,5 veces) y la elevación en la velocidad de sedimentación (6% por unidad). La regresión logística predijo el resultado en el 76,9% de los casos que acabaron y en el 92,3% de los casos que no acabaron en amputación.
ConclusiónEl sistema de puntuación calculado utilizando los coeficientes beta de las variables significativas en el modelo de regresión (grados 4–5 de la escala de Wagner, obstrucción en el Doppler y la elevación de la velocidad de sedimentación, como variables clínica, de imagen y analítica respectivamente) predijo correctamente la amputación durante la hospitalización por infección aguda de pie diabético.
Approximately 7.5% of persons with diabetes have a history of previous or active foot ulcer, and the lifetime risk of experiencing this condition is up to 15% in this population.1 Factors that precipitate a skin break or impair healing can lead to foot ulceration, putting the foot at risk. Peripheral neuropathy implies abnormal forces applied to the foot, lack of protective sensation, and deficient sweating, which leads to skin dryness. Ischemia renders the skin less able to withstand these factors. The coexistence of neuropathy, ischemia (the prevalence of peripheral vascular disease among diabetics is 10%), and leukocyte immune function disorders favors the development of severe, extensive infection,2 and infected foot wounds precede about two-thirds of amputations.3 In this regard, diabetic patients have a 10-fold greater risk of being hospitalized for bone and soft tissue infection of the foot than individuals without diabetes,3 and those with infected ischemic lesions have a 9-fold greater chance of undergoing amputation than those without ischemia or infection.4
This study investigates the predictive factors for amputation during hospital treatment of acute diabetic foot infections by comparing the clinical, laboratory, and imaging data at admission in patients who developed criteria for amputation with those who did not.
Material and methodsA retrospective analysis of clinical records was performed in a cohort of 78 consecutive patients with diabetes type II who had not undergone a previous amputation. All patients had been admitted to the Infectious Disease Department or Vascular Surgery Department of Hospital Central de la Defensa Gomez Ulla in Madrid (Spain) over a 42-month period for acute diabetic foot infection. A clinical evaluation including neurological and vascular status was performed daily, and microbiological (culture of purulent drainage, curetted material, or aspiration of unopened abscesses), laboratory, and radiographic evaluations were carried out every 1 to 2 weeks during hospitalization, in keeping with the routine hospital practice. Noninvasive Doppler study was performed at admission, except when the patient presented good pedal and popliteal pulses and an absence of vascular lesions. Patients were assigned to one of the 6 grades (0–5) of the Wagner ulcer classification system.5,6 Diabetic foot ulcers were classified into 3 groups: 1) neuropathic lesions: those present for months, surrounded by callus, occurring on the plantar aspect of a neuropathic foot (foot warm and well perfused with palpable pulses, decreased sweating, and sometimes, dry skin prone to fissuring), 2) ischemic lesions: those in an ischemic foot in the absence of neuropathy, and 3) mixed or neuro-ischemic lesions: presence of neuropathy and ischemia (foot cool, pulseless, presenting thin, shiny skin with no hair).
Infection was defined by the presence of at least 3 of the following signs or symptoms: fever (≥38°C), leukocytosis (white blood cell count ≥10×103/mm3), local pain, tenderness, erythema, induration and/or presence of purulent secretion. Osteomyelitis was diagnosed by clinical criteria (bone exposure), radiological criteria (decrease in radiological density or cortical lyses in bone subjacent to soft tissue infection), and/or nuclear bone scan criteria (using technetium-99m methylene diphosphonate, gallium Ga 67 citrate, and indium-111-labeled white blood cells as tracers). Patients who underwent amputation at admission, pregnant patients, and those with life-threatening diseases (shock, hemodynamic alterations) or severe immunosuppression (oncological treatments) were excluded.
In accordance with the hospital treatment protocol, patients were parenterally treated for at least 2 weeks with a third-generation cephalosporin plus metronidazole, or a quinolone plus clindamycin, or aztreonam plus clindamycin, together with concomitant surgical debridement, revascularization (bypass), sympathectomy, and/or reconstruction (skin graft) techniques. Patients who developed criteria for amputation during hospital treatment for diabetic foot infection underwent this type of surgery.
For the data analysis, patients were divided into 2 groups: those who ultimately underwent amputation and those who did not. Clinical/epidemiological, laboratory, imaging, and treatment variables were comparatively analyzed between these 2 groups. The clinical/epidemiological variables recorded were gender, age, insulin use, duration of diabetes mellitus, diabetes comorbidities, previous foot lesions, main cause of foot wound, wound location, type of lesion, suppuration, odor, and lesion depth according to the Wagner classification. The laboratory data included white blood cell and platelet counts, hemoglobin, sedimentation rate, glycemia, creatinine concentration, and baseline microbiological culture. Imaging data consisted of alterations on radiography or nuclear bone scan, and lesion location and severity on arterial Doppler study (patients with normal Doppler features and those in whom Doppler was not performed because of good pedal and popliteal pulses and absence of vascular lesions were considered “normal”). Antimicrobial treatment and concomitant surgical techniques were also compared between groups.
Data expressed in terms of frequency were analyzed using the chi-square test, and data expressed as mean±standard deviation, using the Student t-test, Mann-Whitney test or Wilcoxon test, when appropriate. A significance level of P≤0.05 was chosen.
A logistic regression model was performed with amputation (yes/no) as the dependent variable and factors showing significant differences in the bivariate analysis as independent variables. The statistical analysis was performed with SPSS v14 (SPSS Inc., Chicago IL), choosing the “Introduce” command for the regression model. A scoring system was developed to predict amputation, using beta coefficients for significant variables in the regression analysis. The score was validated by receiver operating characteristic (ROC) curve analysis, determining the sensitivity and specificity for each cut-off. The greater the area of the ROC curve covering the theoretical square area, the greater the predictive capability.
ResultsThe demographic, clinical and laboratory data at admission of patients included in this study, divided into the 2 analysis groups, are shown in Table 1. In the overall study population, mean age was 68.9±10.4 years, 57.7% were males, mean time of diabetes evolution was 14.9±8.5 years, and 50.0% of patients were receiving insulin treatment. There were no differences in these parameters when patients were divided into the 2 analysis groups.
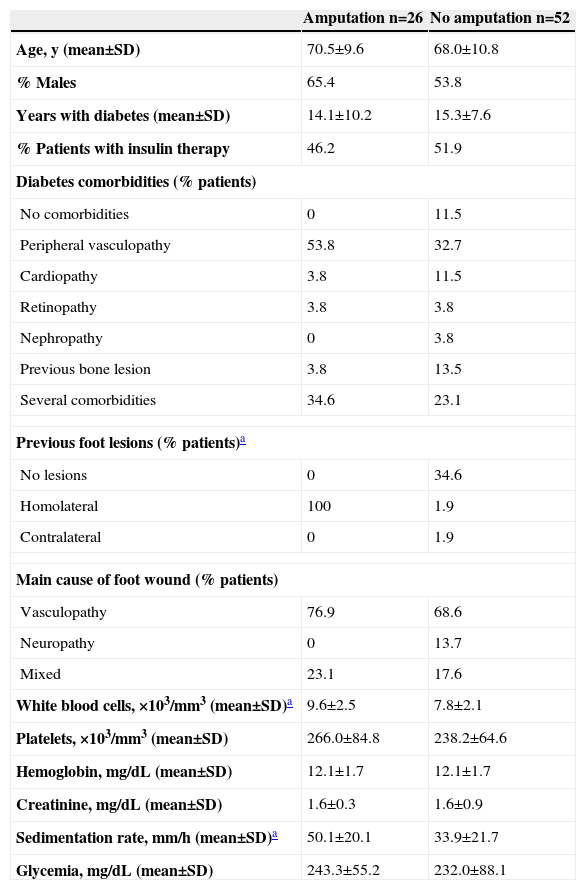
Demographic data, previous lesions, main cause of foot wound, and baseline laboratory data of patients with diabetic foot infection undergoing amputation vs. no amputation
| Amputation n=26 | No amputation n=52 | |
| Age, y (mean±SD) | 70.5±9.6 | 68.0±10.8 |
| % Males | 65.4 | 53.8 |
| Years with diabetes (mean±SD) | 14.1±10.2 | 15.3±7.6 |
| % Patients with insulin therapy | 46.2 | 51.9 |
| Diabetes comorbidities (% patients) | ||
| No comorbidities | 0 | 11.5 |
| Peripheral vasculopathy | 53.8 | 32.7 |
| Cardiopathy | 3.8 | 11.5 |
| Retinopathy | 3.8 | 3.8 |
| Nephropathy | 0 | 3.8 |
| Previous bone lesion | 3.8 | 13.5 |
| Several comorbidities | 34.6 | 23.1 |
| Previous foot lesions (% patients)a | ||
| No lesions | 0 | 34.6 |
| Homolateral | 100 | 1.9 |
| Contralateral | 0 | 1.9 |
| Main cause of foot wound (% patients) | ||
| Vasculopathy | 76.9 | 68.6 |
| Neuropathy | 0 | 13.7 |
| Mixed | 23.1 | 17.6 |
| White blood cells, ×103/mm3 (mean±SD)a | 9.6±2.5 | 7.8±2.1 |
| Platelets, ×103/mm3 (mean±SD) | 266.0±84.8 | 238.2±64.6 |
| Hemoglobin, mg/dL (mean±SD) | 12.1±1.7 | 12.1±1.7 |
| Creatinine, mg/dL (mean±SD) | 1.6±0.3 | 1.6±0.9 |
| Sedimentation rate, mm/h (mean±SD)a | 50.1±20.1 | 33.9±21.7 |
| Glycemia, mg/dL (mean±SD) | 243.3±55.2 | 232.0±88.1 |
The main cause of the foot wound was peripheral vasculopathy (70.5% patients), with no significant differences between the groups. However, a larger percentage of patients with peripheral vasculopathy (53.8% vs. 32.7%) and comorbid conditions (34.6% vs. 23.1%) were seen in the group ultimately requiring amputation than in the group that did not, although the differences were not statistically significant. A significantly higher percentage of patients had previous foot lesions (homolateral lesions in all cases) (P=0.001), higher leukocytosis (P=0.001), and higher sedimentation rate (P=0.002) in the group ultimately undergoing amputation.
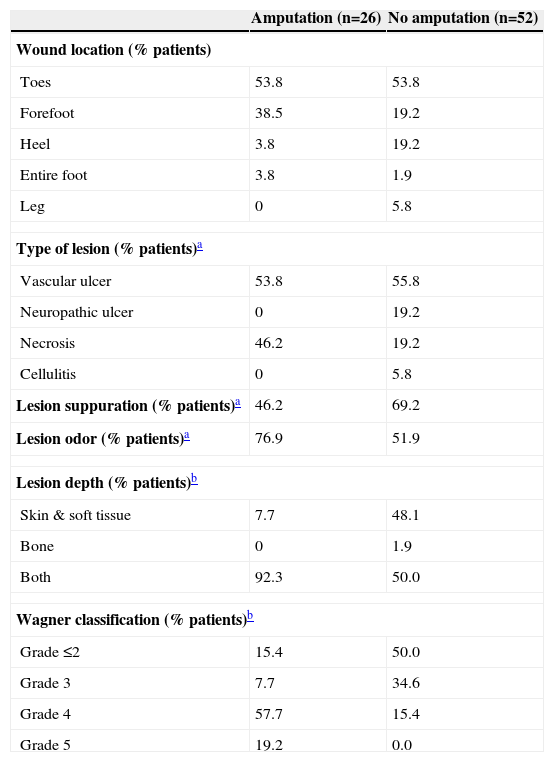
The characteristics of the lesions at admission are shown in Table 2. The most frequent wound location in both groups was the toes (53.8%). A significantly larger percentage of patients presenting necrosis (myonecrosis [gangrene] and/or necrotizing fasciitis), odor, and involvement of skin and soft tissue plus bone was found in the group requiring amputation, whereas a higher percentage of patients with suppuration and exclusive involvement of either bone or skin and soft tissue was found in the group that did not require amputation. Distribution of patients by Wagner ulcer classification showed grades 4 and 5 in 76.9% patients who underwent amputation and grade ≤3 in 84.6% of those who did not (P<0.001).
Characteristics of lesions in patients with diabetic foot infection undergoing amputation vs. no amputation
| Amputation (n=26) | No amputation (n=52) | |
| Wound location (% patients) | ||
| Toes | 53.8 | 53.8 |
| Forefoot | 38.5 | 19.2 |
| Heel | 3.8 | 19.2 |
| Entire foot | 3.8 | 1.9 |
| Leg | 0 | 5.8 |
| Type of lesion (% patients)a | ||
| Vascular ulcer | 53.8 | 55.8 |
| Neuropathic ulcer | 0 | 19.2 |
| Necrosis | 46.2 | 19.2 |
| Cellulitis | 0 | 5.8 |
| Lesion suppuration (% patients)a | 46.2 | 69.2 |
| Lesion odor (% patients)a | 76.9 | 51.9 |
| Lesion depth (% patients)b | ||
| Skin & soft tissue | 7.7 | 48.1 |
| Bone | 0 | 1.9 |
| Both | 92.3 | 50.0 |
| Wagner classification (% patients)b | ||
| Grade ≤2 | 15.4 | 50.0 |
| Grade 3 | 7.7 | 34.6 |
| Grade 4 | 57.7 | 15.4 |
| Grade 5 | 19.2 | 0.0 |
Samples for microbiological culture were taken in 56 patients, and 50 of them (89.2%) yielded bacterial growth. The infection was monomicrobial in 38% of cases (19 of 50), and polymicrobial in 62% of cases (31 of 50). Staphylococcus aureus was the most frequently isolated microorganism (40% of samples), followed by Staphylococcus epidermidis (19%), Klebsiella spp (8%), Pseudomonas aeruginosa (8%), Escherichia coli (6%), Enterococcus spp. (6%) and anaerobes (6%).
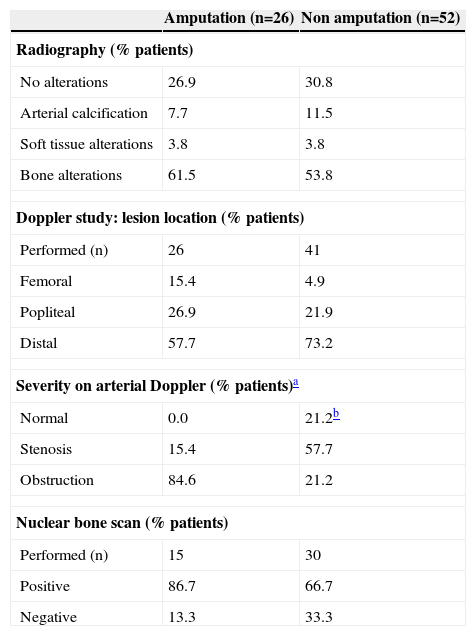
Data on the imaging diagnosis and arterial Doppler at admission are shown in table 3. In the overall population, radiographs disclosed bone alterations in 56.4% patients and no alterations in 29.5%, with no differences when patients were divided into the 2 study groups. Nuclear bone scan was performed in 45 patients and 73.3% were positive, with no significant differences between the groups. Doppler study was carried out in 67 (85.9%) patients: 84.6% of those in the amputated group presented arterial obstruction, whereas stenosis was more frequent (57.7%) in the group without amputation (P<0.001).
Image diagnosis and arterial Doppler in patients with diabetic foot infection undergoing amputation vs. non amputation
| Amputation (n=26) | Non amputation (n=52) | |
| Radiography (% patients) | ||
| No alterations | 26.9 | 30.8 |
| Arterial calcification | 7.7 | 11.5 |
| Soft tissue alterations | 3.8 | 3.8 |
| Bone alterations | 61.5 | 53.8 |
| Doppler study: lesion location (% patients) | ||
| Performed (n) | 26 | 41 |
| Femoral | 15.4 | 4.9 |
| Popliteal | 26.9 | 21.9 |
| Distal | 57.7 | 73.2 |
| Severity on arterial Doppler (% patients)a | ||
| Normal | 0.0 | 21.2b |
| Stenosis | 15.4 | 57.7 |
| Obstruction | 84.6 | 21.2 |
| Nuclear bone scan (% patients) | ||
| Performed (n) | 15 | 30 |
| Positive | 86.7 | 66.7 |
| Negative | 13.3 | 33.3 |
No between-group differences were found with respect to antibiotic treatment: 20%, 40%, and 40% patients ending in amputation, and 23%, 50%, and 27% patients who did not, received third-generation cephalosporin plus metronidazole, aztreonam plus clindamycin, and quinolone plus clindamycin, respectively. Mean duration of treatment was 29.2±7.4 days, with no differences between the groups. With respect to concomitant surgical procedures, debridement, sympathectomy, bypass, and skin graft were performed in 88.5%, 3.8%, 3.8%, and 3.8% of patients ending in amputation and 90.4%, 1.9%, 3.8%, and 3.8% of the other patients, respectively. Mean time to amputation was 18.0±6.6 days.
In the multivariate analysis, Wagner classification grades 4 and 5 showed a higher odds ratio (20.00, 95% CI 3.62–111.11; P=0.001) than reference grades 0–3, which was followed by arterial obstruction on Doppler (odds ratio=12.50; 95% CI=1.42–66.67; P=0.003), and the sedimentation rate (odds ratio=1.06; 95% CI=1.01–1.10; P=0.013). These 3 variables explained the best goodness of the fit (r2 Cox=0.522; P<0.001).
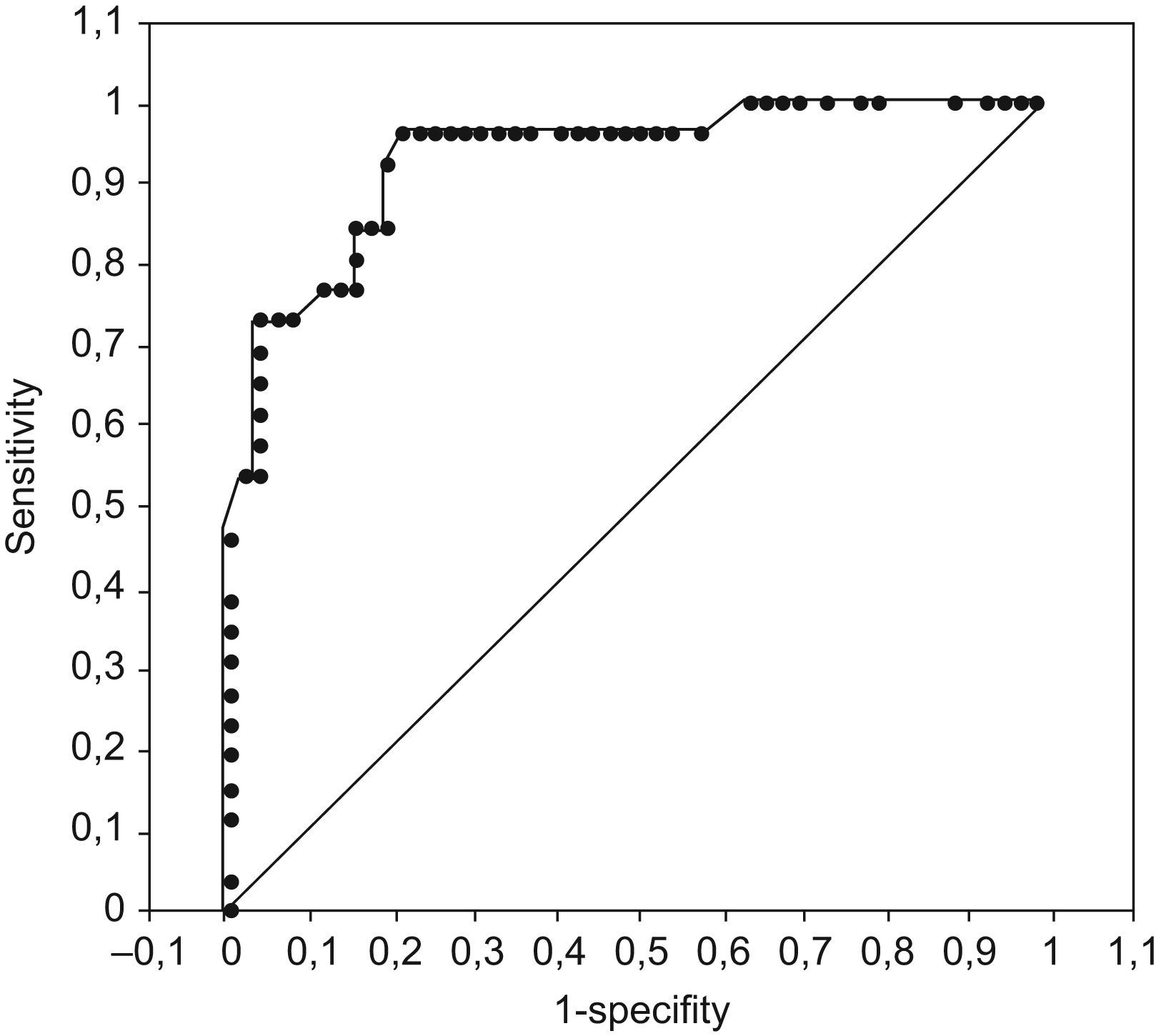
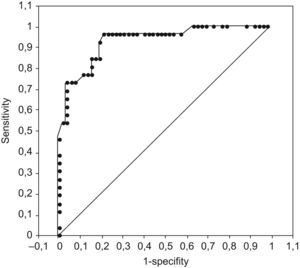
Using exponentiation of the beta coefficients for significant variables (Doppler severity, DS [obstruction=3, stenosis/normal=1], Wagner grade, WG [grades 4 and 5=5; grades 0–3=1], and sedimentation rate, SR [100/crude sedimentation rate]) in the regression analysis, the predictive score was constructed as SCORE=DS+WG+SR. The score range was 2.01–8.78, and the area under curve in the ROC analysis was 0.93 (P<0.001) (Fig. 1).
A cut-off ≤2.31 showed 100% specificity and 37.5% sensitivity for no amputation, and a cut-off ≥8.38 showed 100% specificity and 46.2% sensitivity for amputation. The highest predictive value for amputation was obtained with a predictive score cut-off of 4.375 (sensitivity=96.2%; specificity=78.8%).
DiscussionInfection is rarely implicated in the etiology of diabetic foot ulcers, but ulcers are susceptible to infection and for most of these patients, infection is a pivotal event that leads to limb complications, including amputation. Wound infections must be diagnosed clinically,7 and many risk factors for foot ulcers are also predisposing factors for amputation,8,9 at a site where culture of wound material is usually not recommended10 and radiography is not a highly sensitive indicator of acute bone infection.11 In addition, vascular status should always be assessed because lesions that are ischemic and infected carry a risk of lower limb amputation 90 times greater than lesions without these two factors.4 With respect to vasculopathy, in the current study, data came from Doppler examination in 85.9% cases, in accordance with hospital-based treatment for patients with this condition (70.5%) admitted for acute foot infection, in contrast to other techniques, such as the ankle-brachial index (ABI) or transcutaneous oxygen tension (TcPO2), used in other diabetic populations.12–14
In a 2-year longitudinal outcome study in 1666 consecutive diabetic patients, foot infection carried a 154.5-fold increase in the risk of amputation.3 The long-term prognostic determinants of foot infection identified in diabetic patients were wounds that penetrated to bone (odds ratio=6.7), wounds lasting >30 days (odds ratio=4.7), wound recurrence (odds ratio=2.4), traumatic etiology (odds ratio=2.4), and peripheral vascular disease (odds ratio=1.9).3 In other studies investigating factors associated with a risk of limb loss in patients with diabetic foot problems (not all of whom presented infection), neuropathy, vascular disease, ABI <0.8, gangrene, and infection were significantly associated with limb loss along the study period in the univariate analysis, but only vascular disease and infection remained in the stepwise logistic regression analysis.12 Thus, information on the factors predicting short-term amputation during hospital treatment of patients admitted for acute diabetic foot infections (occurring mainly in those with vascular disease) is of interest for clinicians managing the acute episode, and for this reason, this study focused on identifying these factors.
Variables such as age, gender, years with diabetes, comorbidities, glycemia or insulin use, wound location, and radiography and nuclear bone scan data did not differ between patients ultimately undergoing amputation and those who did not; therefore, these factors were not predictive of short-term amputation during hospital management of acute diabetic foot infections. Nor were there differences between the groups in the microbiological data, which showed a high rate of polymicrobial cultures and S. aureus as the most frequent isolate, as has been previously reported.15,16 In the bivariate analysis, white blood cell count, previous homolateral foot lesions, odor, lesion depth, sedimentation rate, Wagner classification grade, and arterial obstruction on Doppler study were significantly higher in patients ending in amputation. Nonetheless, in the multivariate analysis, the risk of amputation was only increased by Wagner's grade 4 and 5 (20-fold higher risk), obstruction (but not stenosis) seen on arterial Doppler (12.5-fold higher risk) and elevated sedimentation rate (6% risk increase per sedimentation unit). Of note, most lesions in our study population were of vasculopathic origin, and neuropathic lesions occurred in only 13.7% of non-amputated patients. This is consistent with hospital management of these lesions, since ischemia portends a poor prognosis for healing without vascular interventions.9 However, we found no differences between the concomitant surgical procedures performed in patients ultimately requiring amputation and those who did not.
Although this is a retrospective analysis in only 78 consecutive patients, the logistic regression correctly predicted outcome in 87.2% of patients (68 out of 78) in the cohort: 92.3% of those (48 out of 52) who did not undergo amputation and 76.9% of those (20 out of 26) requiring amputation.
As has been reported, amputation is a marker of diabetic foot infections as well as a marker of disease management. High amputation rates can depend on the prevalence of disease, late presentation, inadequate resources, and a particular local approach by surgeons, whereas low amputation rates may be a result of better care, perhaps provided by an experienced “foot team”17 or, conversely, of inappropriate conservative treatment.1 Regardless of these epidemiological/sociological facts, the disease presentation (clinical, imaging, and laboratory data) in patients without a previous amputation is an important clue to predict amputation during hospital management of acute diabetic foot infections on a single-hospital basis, since management of diabetic foot ulcers is based more on opinion than scientific facts.1 In this line, the score calculated in the present study using beta coefficients for the 3 significant variables in the regression model (Wagner grades 4 and 5, arterial obstruction on Doppler, and elevated sedimentation rate as the clinical, imaging and laboratory data, respectively) correctly predicted amputation during hospital management of acute diabetic foot infections.
This study did not receive public or private financial support.
The study was approved by the Ethical Review Committee of Hospital Central de la Defensa Gómez Ulla, Madrid, Spain.
Conflict of interestThe authors have no conflict of interest to declare.
A part of this study was accepted for presentation at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington DC, October 2008.