The aim of this study was to analyze the presence of antibodies against both Yamagata and Victoria influenza B lineages and to check the response after seasonal trivalent vaccination.
Materials and methodsHaemagglutination inhibition assays were performed with pre-and post-vaccination serum samples from 174 individuals ≥65 years of age vaccinated with seasonal trivalent influenza vaccines during the 2006–2007, 2008–2009, 2009–2010 and 2010–2011 vaccine campaigns.
Results33.9% of individuals showed pre-vaccine protective antibodies (≥1/40) against B/Yamagata lineage and 41.4% against B/Victoria lineage. The annual trivalent vaccine induced significant homologous seroconversion in 14–35.6% of individuals in each vaccine campaign.
ConclusionsThe population ≥65 years has low-moderate seroprotection against B influenza lineages. Trivalent vaccination induced a slight increase of seroprotection. The trivalent vaccine should be administered to all individuals ≥65 years in all vaccine campaigns.
El objetivo de este estudio es analizar la presencia de anticuerpos frente a los linajes de gripe B Yamagata y Victoria y comprobar la respuesta frente a la vacuna trivalente estacional.
Material y métodosSe realizó ensayo de inhibición de hemaglutinación con sueros pre- y posvacunales de 174 individuos≥65 años vacunados con vacuna trivalente estacional en las campañas vacunales de 2006-2007, 2008-2009, 2009-2010 y 2010-2011.
ResultadosEl 33,9% de los individuos mostraron anticuerpos prevacunales protectores (≥1/40) frente al linaje B/Yamagata y el 41,4% frente al linaje B/Victoria. La vacuna trivalente anual indujo seroconversión homóloga significativa en el 14-35,6% de los individuos en cada temporada.
ConclusionesLa población≥65 años posee una baja-moderada seroprotección frente a los linajes de gripe B. La vacunación trivalente indujo un leve incremento de la seroprotección. Es necesaria la administración de la vacuna trivalente en todas las campañas en los≥65 años.
Influenza B infection is commonly misconstrued as a milder disease than A type influenza infection.1 However, current studies demonstrate that influenza B cause epidemics with remarkable percentage of people needing hospitalization similarly to A type infections.1 Vaccination is still the best method for limiting influenza transmission and clinical complications, but one of the biggest problems of the currently used Trivalent Inactivated Influenza Vaccines (IIV3s) is the lower humoral response induced against B type influenza viruses than A type viruses,2 and also the mismatch between the circulating influenza B lineage and that was included in the vaccine composition.3 This mismatch between B influenza lineages is a common issue that occurs each 2–4 years approximately. Most of the currently used influenza vaccines have trivalent composition, and in the case of an existing mismatch the usefulness of the vaccine is limited against the B influenza lineage not included in their composition. Thus, new designs of influenza vaccines as Quadrivalent Influenza Vaccine (QIV) include 4 different viruses A and B viruses in their composition. The aim of this study is to describe the presence of antibodies (Abs) against both B influenza lineages (Yamagata and Victoria) before vaccination in a Spanish elderly population, and the humoral response after IIV3s administration during 4 different influenza vaccine campaigns (IVC), two of them presenting mismatch.
Material and methodsAn experimental study was designed recruiting 174 patients ≥65 years from Sentinel Surveillance Network of Castile and Leon (Spain) during 2006–2007 (n1=45), 2008–2009 (n2=43), 2009–2010 (n3=43) and 2010–2011 (n4=43) IVC. Pre-vaccine serum samples were obtained before IIV3 administration and post-vaccine serum samples after 28 days. The IIV3s administered contained the influenza B strains recommended by WHO for the North Hemisphere4–7: 2006–2007, B/Malaysia/2506/2004-like (B/Victoria lineage); 2008–2009, B/Florida/4/2006-like (B/Yamagata lineage); 2009–2010, B/Brisbane/60/2008-like (B/Victoria lineage); 2010–2011, B/Brisbane/60/2008 (B/Victoria lineage). During 2006–2007 and 2008–2009 IVC a mismatch was observed between the influenza B lineages included in IIV3s and those mainly circulated (2006–2007: B/Jiangsu/10/2003-B/Yamagata; 2008–2009: B/Brisbane/60/2008-B/Victoria). Serum samples were stored at −20°C until their use. The 2007–2008 IVC was not included in this study due to deficient storage conditions. The B viruses strains used were obtained from the “WHO Influenza Reagent Kit for Identification of Influenza Isolates”. Those kits were supplied by International Reagent Resource (Manassas, VA, USA) during the IVC analyzed. The analysis of the presence of haemaglutinant Abs against both influenza B lineages was performed by haemaglutination inhibition assay (HAI).8 HAI was performed following the manual for the laboratory diagnosis and virological surveillance of Influenza,8 but using specific conditions as 0.75% diluted hens red blood cells (RBCs) that do not modifies the standardized protocol. This test was performed in 96-microV well plates. Previously to the HAI the serum samples were treated with RDE (Receptor Destroying Enzime; Denka Seiken, Tokyo, Japan) and then adsorbed with the previous described hen RBCs for eliminating the unspecific inhibitors from blood. Statistical analysis was performed using classical European Medicament Agency (EMA) criteria for the evaluation of vaccine efficacy.9 Those criteria established that populations ≥60 years must show a Seroprotection rate (SPR) ≥60%, a seroconversion rate (SCR) ≥30%, and a Geometric Mean Titers (GMT) increase between pre and post-vaccine serum samples ≥2.0. The negative results in HAI were assumed as half of the detection value (1/10). A titer ≥1/40 was considered as protective.10 Seroconversion was defined as an increase of at least four-fold titers between pre and post-vaccination serum samples. Non-parametric McNemar test was used to evaluate the seroconversion by using SPSS V20 (IBM, Armonk, NY, USA). Statistical significance was taken at the p<0.05 value.
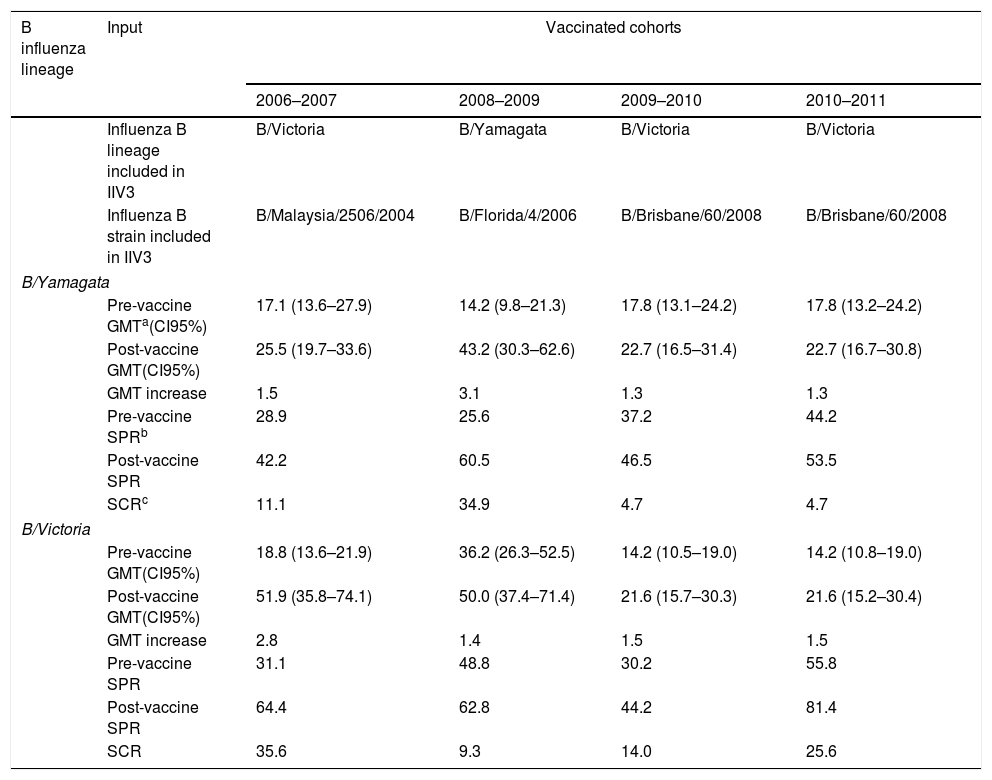
ResultsGlobally, 33.9% of individuals showed pre-vaccination protective titers of Abs (≥1/40) against B/Yamagata lineage and 41.4% against B/Victoria lineage. After IIV3s administration, 50.6% of individuals showed protective Abs against B/Yamagata lineage and 63.2% against B/Victoria lineage. IIV3s induced a significant homologous response against B/Victoria lineage in 2006–2007 (McNemar; p=0.000), 2009–2010 (McNemar; p=0.031) and 2010–2011 (McNemar; p=0.001) IVCs, and also against B/Yamagata lineage in 2008–2009 IVC (McNemar; p=0.000). Vaccination against B/Victoria lineage (B/Malaysia/2506/2004-like strain) during 2006–2007 IVC induced a low but significant heterotypic response against B/Yamagata lineage (McNemar; p=0.008). This cross-immune reaction was not present against B/Victoria lineage when elderly people were vaccinated against B/Yamagata lineage (2008–2009 IVC). Pre and post-vaccination GMT, SPR and SCR values are described in Table 1. The SPR, SCR and GMT increase were higher than 60%, 30% and 2.0 respectively against B/Yamagata lineage only in 2008–2009 IVC. Against B/Victoria lineage, SPR was higher than 60% in 2006–2007 (64.4%), 2008–2009 (62.8%) and 2010–2011 (81.4%) IVCs, while SCR and GMT increase were higher than 30% and 2.0 respectively only in 2006–2007 IVC (35.6% and 2.8).
Values of pre and post-vaccine geometric mean titers, seroprotection rate, seroconversion rate and geometric mean titers increase against B influenza lineages after trivalent influenza vaccination of the elderly population.
| B influenza lineage | Input | Vaccinated cohorts | |||
|---|---|---|---|---|---|
| 2006–2007 | 2008–2009 | 2009–2010 | 2010–2011 | ||
| Influenza B lineage included in IIV3 | B/Victoria | B/Yamagata | B/Victoria | B/Victoria | |
| Influenza B strain included in IIV3 | B/Malaysia/2506/2004 | B/Florida/4/2006 | B/Brisbane/60/2008 | B/Brisbane/60/2008 | |
| B/Yamagata | |||||
| Pre-vaccine GMTa(CI95%) | 17.1 (13.6–27.9) | 14.2 (9.8–21.3) | 17.8 (13.1–24.2) | 17.8 (13.2–24.2) | |
| Post-vaccine GMT(CI95%) | 25.5 (19.7–33.6) | 43.2 (30.3–62.6) | 22.7 (16.5–31.4) | 22.7 (16.7–30.8) | |
| GMT increase | 1.5 | 3.1 | 1.3 | 1.3 | |
| Pre-vaccine SPRb | 28.9 | 25.6 | 37.2 | 44.2 | |
| Post-vaccine SPR | 42.2 | 60.5 | 46.5 | 53.5 | |
| SCRc | 11.1 | 34.9 | 4.7 | 4.7 | |
| B/Victoria | |||||
| Pre-vaccine GMT(CI95%) | 18.8 (13.6–21.9) | 36.2 (26.3–52.5) | 14.2 (10.5–19.0) | 14.2 (10.8–19.0) | |
| Post-vaccine GMT(CI95%) | 51.9 (35.8–74.1) | 50.0 (37.4–71.4) | 21.6 (15.7–30.3) | 21.6 (15.2–30.4) | |
| GMT increase | 2.8 | 1.4 | 1.5 | 1.5 | |
| Pre-vaccine SPR | 31.1 | 48.8 | 30.2 | 55.8 | |
| Post-vaccine SPR | 64.4 | 62.8 | 44.2 | 81.4 | |
| SCR | 35.6 | 9.3 | 14.0 | 25.6 | |
The results of our study show that previously to vaccination, the elderly population presented protective Abs against B/Victoria lineage (41.4%) in a higher percentage than B/Yamagata lineage (33.9%), and also that vaccination with IIV3s increased seroprotection in a percentage ranging 20% against both lineages. The differences found in pre-vaccination seroprotection between both lineages may be caused by the priming history of the people studied. Some studies show that the presence of Abs against B influenza viruses in children and young people is significantly higher against B/Yamagata lineage than B/Victoria.11 This may be caused because elderly population has been probably primed during their childhood with viruses related to B/Lee/40 strain, phylogenetically closer to the current B/Victoria lineage than B/Yamagata.12 However, children has been probably primed by strains drifted from B/Yamagata/16/88 virus, suggesting a recall response named as antigenic sin or back-boosting.13
IIV3s induced a significant homologous response against the lineage included in vaccine in all IVC analyzed, but the SCR reached was low-moderate for both B/Victoria lineage (14–35.6%) and B/Yamagata lineage (34.9%). Despite this limited response, the seropotection reached after vaccination ranges 42.2–81.4% depending on each IVC, so the pre-vaccine Abs levels keep moderate-high herd immunity against both influenza B lineages. Analyzing our data using classical EMA criteria showed that the homologous response against B/Victoria lineage was only effective during 2006–2007 IVC (SPR: 64.4; SCR: 35.6; GMT increase: 2.8), who were vaccinated against B/Malaysia/2506/2007-like strain. The others IVC vaccinated against B/Brisbane/60/2008-like strain did not show an effective humoral response. Our data show that influenza vaccination against B/Victoria lineage failed during 2009–2010 and 2010–2011 IVC, despite that no mismatch was addressed to this influenza season. This seems that the response to vaccination is dependent on the antigenicity features of the strain included in vaccine, and demonstrates the importance of the correct selection of strains by WHO. Despite this vaccine failure, during 2010–2011 SPR against B/Victoria lineage reached 81.4%, demonstrating the importance of the pre-vaccine Abs present in the elderly population. Vaccination with B/Florida/4/2006-like strain (B/Yamagata lineage) in 2008–2009 IVC induced an effective humoral response (SPR: 60.5; SCR: 34.9; GMT increase: 3.1). However, we were not able to study differences between vaccination with different B/Yamagata strains because we include only one vaccinated group against this virus in our study.
We observed heterotypic seroconversion against the lineage not include in IIV3s in all IVC, with SCR ranging 4–11%. This heterotypic response was only significant in 2006–2007 IVC against B/Yamagata lineage when the elderly people were vaccinated against B/Victoria lineage (B/Malaysia/2506/2007-like strain). This type of heterotypic responses are not frequent, but has been previously observed in other works.14,15 Despite this interesting issue, those heterotypic reactions were far from being effective, so IIV3s vaccination is not useful to protect elderly people against the lineage not included in the vaccine. Two of the IVC studied suffered a mismatch between the lineage included in vaccine and the lineage that mainly circulate during the influenza season (2006–2007 and 2008–2009). Our results showed that the SPR reached after vaccination against the lineage not included in IIV3s was 42.2% in 2006–2007 (B/Yamagata lineage) and 62.8% in 2008–2009 IVC (B/Victoria lineage). Because the IIV3s have limited capacity for inducing a heterotypic response, the results of our study show that during IVC presenting mismatch, the immunity against the lineage not included in the vaccine is almost exclusively dependent on the background protection present before vaccination.
In summary, a great percentage of elderly population does not have protective Abs against any of influenza B lineages, Yamagata or Victoria. Seroprotection reached after IIV3s administration was moderate in some IVC. The seroprotection reached after vaccination by all elderly populations studied largely depends on the presence of the protective Abs present previous to vaccination, so to mitigate the low-moderate efficacy of IIV3s commonly used, the elderly population may be vaccinated yearly. Use of QIV could be useful because of the limited cross-immunity observed between both influenza B lineages and to prevent the mismatch in some influenza epidemics.16
FundingThis work did not receive funding of any kind.
Conflict of interestsThe authors declare no conflict of interests.