The results of a study on the household contacts of patients with D. fragilis infection are presented.
MethodsA prospective, descriptive study was carried out on all Dientamoeba fragilis-infected patients treated at the Tropical Medicine Unit of Hospital Universitario Central de Asturias between 2012- 2017 and their household contacts. Three stool samples per patient and three stool samples from each of their household contacts were concentrated and analysed. Polymerase chain reaction (PCR) was used to detect the presence of D. fragilis in all stool samples. Co-infection with E. vermicularis was studied in both groups. Patients and contacts who failed to deliver one or more samples for diagnosis and patients without household contacts were excluded.
Results44 Patients infected with D. fragilis, as well as their 97 household contacts were enrolled. 50.5% of household contacts had a positive PCR for D. fragilis. 20 were also coinfected with E. vermicularis. The presence of infection was significantly more frequent in patients with children (34/15 versus 24/24; p= 0.064; OR: 2.267 [0.988-5.199]), E. vermicularis infection in the children being 20/29 versus 0/48 (p=0.0001), and in another family member being 29/20 versus 15/33 (p=0.008; OR: 3.190 [1.384-7.352]).
ConclusionsThe prevalence of D. fragilis infection in household contacts was high. It was associated with the presence of children in the family nucleus and coinfection with E. vermicularis irrespective of gender, age, rural area or contact with animals.
Se presentan los resultados de un estudio de contactos domiciliarios de pacientes con infección por Dientamoeba fragilis (D. fragilis).
MétodosEstudio prospectivo descriptivo realizado en todos los pacientes diagnosticados de infección por D. fragilis atendidos en la Unidad de Medicina Tropical del Hospital Universitario Central de Asturias entre 2012-2017 y sus contactos domiciliarios. Se analizaron 3 muestras de heces concentradas tanto para los casos como para sus contactos. La presencia de D. fragilis se confirmó mediante reacción en cadena de la polimerasa (PCR). En ambos grupos se estudió la presencia de coinfección por Enterobius vermicularis (E. vermicularis). Se excluyeron los pacientes y los contactos que no entregaron una o más muestras para el diagnóstico, así como los pacientes sin contactos domésticos.
ResultadosSe incluyeron 44 pacientes infectados por D. fragilis, así como sus 97 contactos domiciliarios. El 50,5% de los contactos tuvo PCR positiva para D. fragilis. Veinte además estaban coinfectados por E. vermicularis. La presencia de infección fue significativamente más frecuente en pacientes con niños (34/15 versus 24/24, p=0,064; OR: 2,267 [0,988-5,199]), infección por E. vermicularis en ellos (20/29 versus 0/48, p=0,0001) o en otro miembro de la familia (29/20 frente a 15/33, p=0,008; OR: 3,190 [1,384-7,352]).
ConclusionesLa prevalencia de infección por D. fragilis en los contactos domiciliarios fue elevada y se asoció con la presencia de niños en el núcleo familiar y la coinfección con E. vermicularis independiente del sexo, edad, zonas rurales o contacto con animales.
Dientamoeba fragilis is a pathogenic protozoan of the human gastrointestinal tract with a worldwide distribution. It has emerged as an important and misdiagnosed cause of chronic gastrointestinal illnesses such as diarrhea and “irritable-bowel-like” gastrointestinal disease.1–4 Recent studies have described a high prevalence of infection in household contacts of infected patients without consensus on the different risk factors.5–6
The results of a research of household contacts of patients with D. fragilis infection with special focus on the clinical and epidemiological aspects are described.
Material and MethodsA prospective and descriptive research which included all the patients with Dientamoeba fragilis infection who were attended for the first time in the Tropical Medicine Unit of Hospital Universitario Central de Asturias between January 2012 and January 2017 and their household contacts, was performed.
An epidemiological questionnaire that included demographic variables such as sex, age, country of origin, international travelling and classical risk factors for parasitic infections (contact with soil, unsafe water, presence of pets or other animals, type of job, travelling, etc.) and a complete physical examination were performed. The clinical history included diarrhea within the preceding three months, nature of the diarrhea, abdominal pain, intensity of fever, nausea and/or vomiting, urticaria, anal pruritus, anorexia, and weight loss. Diarrhea was defined as three or more unformed or liquid stools per day for at least three days. All cases were asked about their household contacts and all household contacts of a positive patient were asked about performing a study of infection by D. fragilis with the same protocol as the one used for cases.
Laboratory AnalysisScreening for all cases comprised blood count and biochemistry included liver enzymes level. Eosinophilia was defined as >500 eosinophils/mm3
Three stool samples per patient and another three stool samples from each of their household contacts were concentrated by using Copropack Extraction Kit C100 (Cromakit, Spain) according to manufacturer's instructions. These were then stained with lugol and screened under a light microscope with a low magnification to detect helminth eggs, protozoan trophozoites and cysts. PCR was used to detect the presence of D. fragilis in the stool samples, which were previously extracted by using QIAmp DNA stool Mini kit (Qiagen, Netherland), with methods based on polymerase chain reaction (PCR) described in previous researches7.
A pinworm test was performed in all cases and household contacts with D. fragilis in stool samples. Thus, pinworm eggs or a few adult worms which had been adhered to a piece of cellophane tape applied to the anal region on 2 consecutive mornings just after the infected person woke up and before any bowel movement or cleansing (bath or shower), were identified by examination under a microscope8.
Cases and contacts who failed to deliver one or more pieces of cellophane tape or stool samples, as well as those who had received recent anti-parasitic treatment, were excluded. For the purpose of this research, cases without household contacts were also excluded.
Ethics StatementThis research was conducted as a part of the project entitled “Useful of molecular diagnosis techniques in Parasitology”, which was validated and approved by the Ethical Committee of Clinical Investigation of Asturias (Spain).
Statistical analysisCategorical variables were described by relative and absolute frequencies. Continuous variables were described as mean and standard deviations under symmetry and by median and range otherwise. Qualitative variables were compared using the Fisher's exact test, the χ2 test, when necessary. In addition, Odds ratio (OR) with 95% of confidence interval were provided in order to describe the size of the observed effects. For quantitative variables, the Student-Welch test for independent variables or the Mann-Whitney U test were used. Significance was designated at p<0.05. A binary logistic regression analysis using a step-wise (Wald) to determine the factors influencing the mortality of the infection was performed. All tests were performed with SPSS 20.0 Package System.
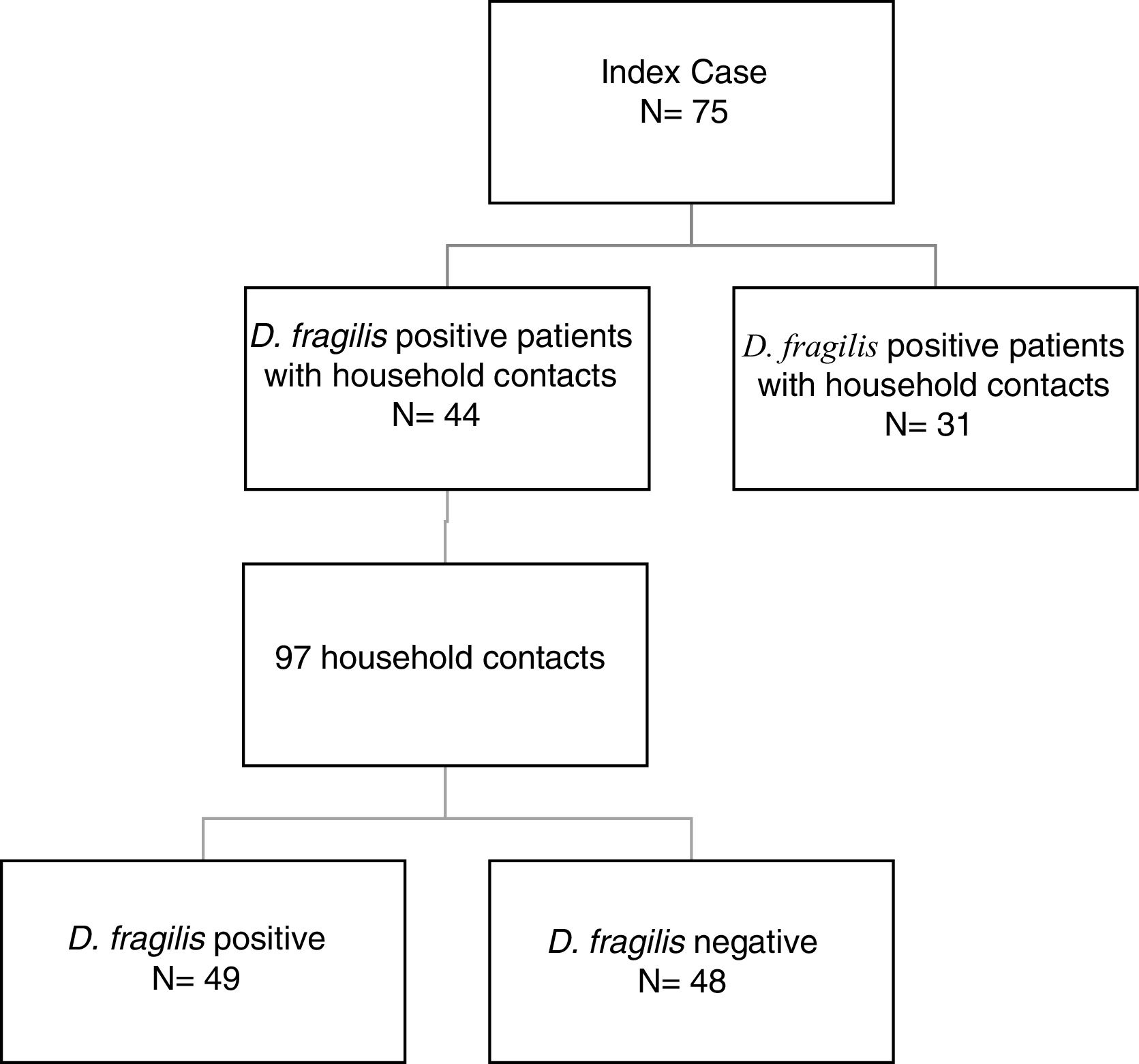
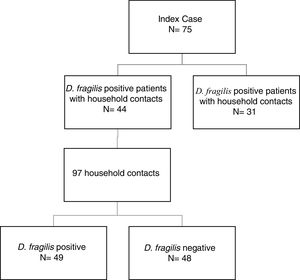
ResultsDuring the period of study, 75 patients were diagnosed of D. fragilis infection with a median age of 35 [18] years (range 5-77), noting that forty of them were female (54.7%). Given it was not possible to perform a contact study, 31 initial cases were excluded. The remaining 44 D. fragilis-infected initial cases and their 97 household contacts were enrolled in the research. (Figure 1).
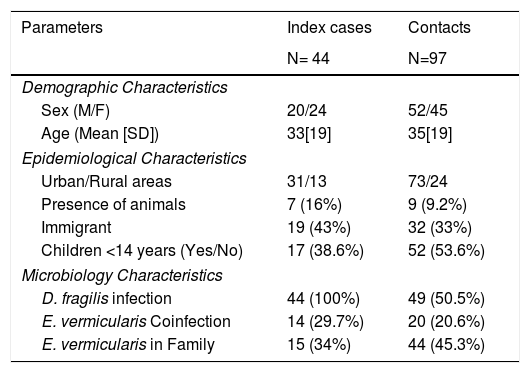
Of those 97 enlisted household contacts, 53% of them were males. The mean age was 35 years [19], and 23 were children under 18 years old. By far, most of the cases came from Spain (67%), followed by Ecuador (9.4%), Equatorial Guinea (8.2%), Colombia (6.2%), Pakistan (5.2%), Paraguay (3%) and Sahara (1%). The average stay in Spain for immigrant population was 1.425 [1.448] days and no cases had travelled to their country of origin in the last year. Fifty-eight contacts (59.8%) had children in the family, 52 of them under 14 years old. Only nine cases had animals at home. Twenty-four lived in rural areas. Forty-nine of the households (50.5%) had a positive PCR for D. fragilis. Twenty contacts (20.6%) had a coinfection with E. vermicularis and 44 had some other member of the family coinfected. The characteristics of index cases and contacts had been described in Table 1.
Characteristics of index and contacts cases.
| Parameters | Index cases | Contacts |
|---|---|---|
| N= 44 | N=97 | |
| Demographic Characteristics | ||
| Sex (M/F) | 20/24 | 52/45 |
| Age (Mean [SD]) | 33[19] | 35[19] |
| Epidemiological Characteristics | ||
| Urban/Rural areas | 31/13 | 73/24 |
| Presence of animals | 7 (16%) | 9 (9.2%) |
| Immigrant | 19 (43%) | 32 (33%) |
| Children <14 years (Yes/No) | 17 (38.6%) | 52 (53.6%) |
| Microbiology Characteristics | ||
| D. fragilis infection | 44 (100%) | 49 (50.5%) |
| E. vermicularis Coinfection | 14 (29.7%) | 20 (20.6%) |
| E. vermicularis in Family | 15 (34%) | 44 (45.3%) |
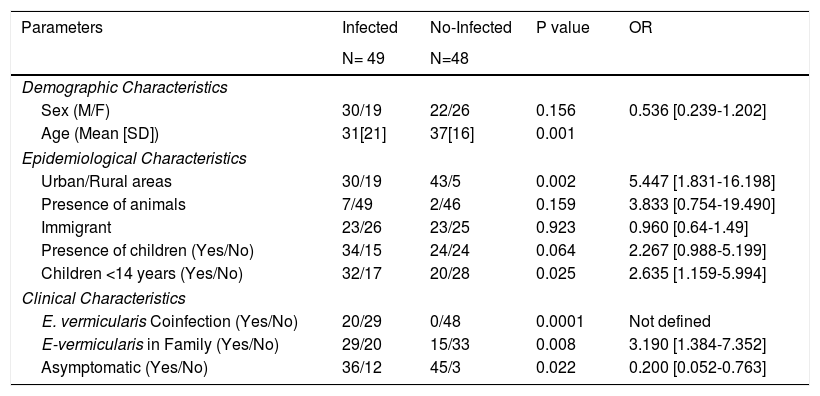
Most cases were males coming from Spain and living in urban areas without animal contact. There was a family enlisted in the study who lived in a rural area and had a history of contact with animals (horses, sheeps, dogs) whose veterinary parasitological studies did not demonstrate the presence of D.fragilis. There were no significant differences in sex, age or immigrant population between infected and not infected contacts. The presence of infection was significantly more frequent in cases from urban areas (p =0.002; OR:5.447 [1.831-16.198]). The characteristics of infected and not infected contacts had showed in Table 2.
Differential characteristics of infected and no-infected contacts.
| Parameters | Infected | No-Infected | P value | OR |
|---|---|---|---|---|
| N= 49 | N=48 | |||
| Demographic Characteristics | ||||
| Sex (M/F) | 30/19 | 22/26 | 0.156 | 0.536 [0.239-1.202] |
| Age (Mean [SD]) | 31[21] | 37[16] | 0.001 | |
| Epidemiological Characteristics | ||||
| Urban/Rural areas | 30/19 | 43/5 | 0.002 | 5.447 [1.831-16.198] |
| Presence of animals | 7/49 | 2/46 | 0.159 | 3.833 [0.754-19.490] |
| Immigrant | 23/26 | 23/25 | 0.923 | 0.960 [0.64-1.49] |
| Presence of children (Yes/No) | 34/15 | 24/24 | 0.064 | 2.267 [0.988-5.199] |
| Children <14 years (Yes/No) | 32/17 | 20/28 | 0.025 | 2.635 [1.159-5.994] |
| Clinical Characteristics | ||||
| E. vermicularis Coinfection (Yes/No) | 20/29 | 0/48 | 0.0001 | Not defined |
| E-vermicularis in Family (Yes/No) | 29/20 | 15/33 | 0.008 | 3.190 [1.384-7.352] |
| Asymptomatic (Yes/No) | 36/12 | 45/3 | 0.022 | 0.200 [0.052-0.763] |
All infected cases described water consumption and plant sanitized. Although a study on potentially contaminated surfaces was not performed, it is worth noting that in the house of one family there was a water tank where the presence of D. fragilis was demonstrated.
The association among the presence of children in the nuclear family, specially those who were less than 14 years old, and an increased risk of D. fragilis infection, was studied. The presence of infection in contacts was significantly higher in those who had had contact with children (34/15 versus 24/24; p= 0.064; OR:2.267[0.988-5.199]), particularly if the children was under 14 years (32/17 versus 20/28; p= 0.025; OR:2.635[1.159-5.994]).
When we studied the relationship between the infection for E. vermicularis and D. fragilis we found that the presence of E. vermicularis in the patient (20/29 versus 0/48; p=0.0001) or in another members of his family (29/20 versus 15/33; p=0.008;OR:3.190[1.384-7.352]) increased in a significantly way the risk of D. fragilis infection.
Multivariable analysis confirmed the relation between the presence of E. vermicularis infection and apparition of D. fragilis in contacts (p= 0.0001)
DiscussionAlthough Dientamoeba fragilis was initially considered nonpathogenic, several publications have shown its pathogenic potential. Nevertheless, questions about its way of transmission and its actual prevalence persist unknown9,10. Thus, in a review of 50 publications the prevalence of D. fragilis ranges from 0.3 to 52% with no consensus on age or gender distribution3. These differences may be due to the different diagnostic techniques applied, obtaining higher prevalence in those researches using methods based on the PCR to detect the parasite11. In Spain, only two studies using conventional parasitological techniques have estimated prevalence rates between 1.5-7% in Catalonia12,13. As far as we know, our research is the first work which study the epidemiological characteristics of this parasite in Spain using a method based on the PCR, the current gold standard.
It is worth noting the high prevalence rate (50.5%) observed among contacts. This prevalence is clearly higher than in any other previous studies14. This data indicates not only the existence of a significant risk of transmission within close contacts but also warn about the significant prevalence of asymptomatic carriers among them.
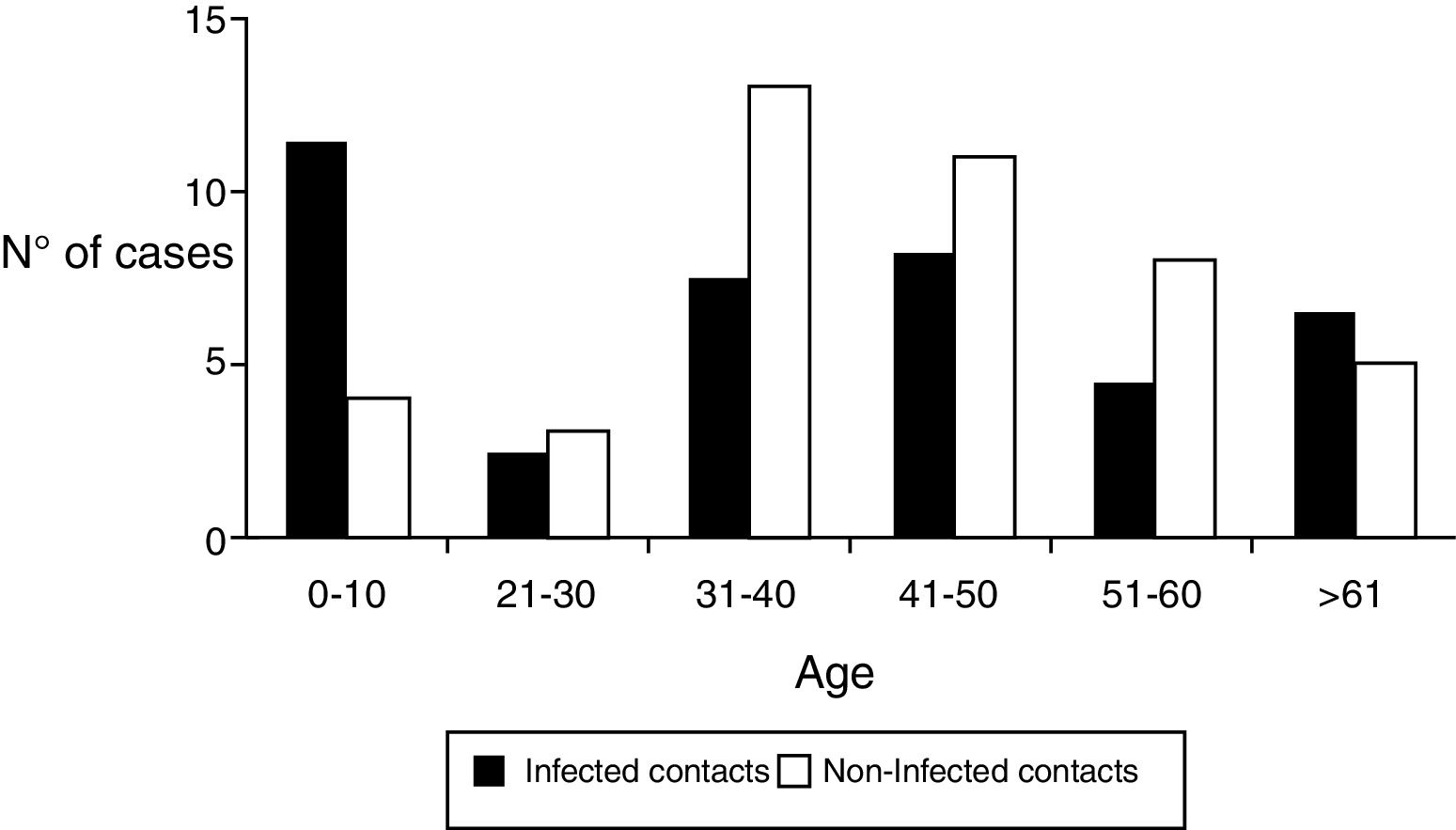
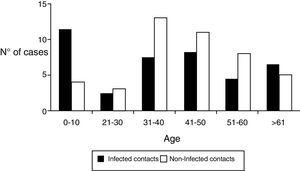
Regarding the demographic characteristics several prospective studies have shown a bimodal peak with a strong association between age and D. fragilis carriage in children (peak at age 7 years) and adults of parental age (peak at age 40 years)3,8,11,15. However, Rayan et al.16, reported the highest incidence of Dientamoeba in people aged 30 to 40 years though this finding was statistically insignificant. In a cohort of patients suspected of suffering from intestinal parasitosis, Stensvold et al.17,found a highest incidence of Dientamoeba in patients aged 16 to 20 years. In our research, the distribution of infected patients had a modal peak around 7-10 years with a minimal second peak in adults about 40 years but without significant differences (Figure 2).
Most reports suggest that females are more likely to harbor Dientamoeba than males16–19 and this fact had been associated to close and frequent contact between young children and their mothers compared to their fathers in some communities.11 Our results showed that the presence of infection in contacts were more frequent in males, although without significant differences. On the other hand, as Rayan et al., 16 we observed how the presence of children in the family nucleus especially under 14 years, increases the risk of infection in adults of parental age. This finding may support that the differences observed between the gender distributions of Dientamoeba in various studies could be linked to the roles of both sexes amongst different cultural groups or societies.
A higher prevalence rate of D. fragilis in patients with low socioeconomic status has been described.11 Nevertheless, this fact is not supported by our data since our patients belonged to medium (immigrant patients) or high (Spanish natives) socioeconomic status.
Despite of several authors have postulated that D. fragilis infection could be a zoonosis associated to different animals, such as pigs20, only nine of the cases in our research had close contact with animals. The remaining patients lived in urban areas neither animal contact nor untreated water.
The transmission of D. fragilis by eggs of Enterobius vermicularis (pinworm) has been repeatedly suggested as a possibility, and it was recently substantiated by the identification of D. fragilis DNA inside pinworm eggs21,22. Girginkardeşler N et al.21, studied the role of E. vermicularis in the transmission of D. fragilis choosing two groups of patients, the first one with E. vermicularis infection (n = 187), and the second one with D. fragilis infection (n = 126). They found that the 9.6% of the patients belonged to the first group (Pinworm Group) were coinfected, while the prevalence of coinfection for the second group (Dientamoeba group) resulted in 25.4%.
In our series, dual infections were observed in all contacts with D. fragilis infection so it is important to note that the presence of E. vermicularis in family nucleus had an important role in the development of infection. The coincidence rates of D. fragilis and E. vermicularis were clearly higher than the prevalence of coinfection expected if there was no association between both agents, and that suggests a common relationship between these two parasites, possibly in the process of entering human body. E. vermicularis infection is usually more common in younger children, indicating that younger children may also involve a higher risk for D. fragilis infection. These findings also raise the question of whether the unrelated symptoms of the pinworm infected patients such as abdominal pain and diarrhea may actually be due to overlooked Dientamoeba infections.
In addition, the lack of an environmental surfaces study can be considered as a limitation for the research. Nevertheless, the presence of D. fragilis in a water tank used by one of the families as well as the recent report of a cystic form support a possible fecal-oral transmission route.
In conclusion the prevalence of D. fragilis infection in household contacts was elevated and was associated to the presence of children in the family nucleus and the coinfection by E. vermicularis independent of sex, age, rural areas or contact with animals.
Our research opens the way to new questions such as the role of E. vermicularis, its transmission mechanism, and the role of the presence of children in the families. Further research with larger number of patients should be performed to solve these questions.
Conflicts of InterestThe authors declare that they have no conflict of interest.