Sexually transmitted infections are a global public health problem both due to their high prevalence and due to their morbidity. A rapid and precise diagnosis is key to establishing appropriate targeted treatment and also to decreasing dissemination of these diseases among the high-risk population. To perform adequate testing for sexually transmitted infections, many of which are asymptomatic, it is necessary to carry out the diagnostic testing according to the clinical and behavioural indicators. The preventive advice must be comprehensive and personalised. The incorporation and improvement of molecular biology techniques is a very useful tool, complementing the classic techniques, such as microscopy and culture. Correct diagnosis will allow for an adequate treatment from the beginning, preventing the possible onset and dissemination of antibiotic resistance, an emerging problem in the current context of sexually transmitted infections.
Las infecciones de transmisión sexual son un problema global de salud pública tanto por su alta prevalencia como por su morbilidad. Un diagnóstico rápido y preciso es clave para la instauración de un tratamiento dirigido adecuado para la disminución de la diseminación de estas patologías entre población de elevado riesgo. Para efectuar un adecuado despistaje de las infecciones de transmisión sexual, con frecuencia asintomáticas, es necesario realizar las pruebas diagnósticas en función de los indicadores clínicos y conductuales. El consejo preventivo debe ser integral e individualizado. La incorporación y mejora de las técnicas de biología molecular son una herramienta muy útil complementando las técnicas clásicas, como microscopía y cultivo. El correcto diagnóstico permitirá un tratamiento adecuado desde un inicio evitando la posible aparición y diseminación de resistencias antibióticas, problema emergente en el contexto actual de las infecciones de transmisión sexual.
Sexually transmitted infections (STIs) are diseases caused by bacteria, viruses, protozoa and ectoparasites which are predominantly sexually transmitted, including by vaginal, oral and anal sex, or direct contact with skin or mucous membranes. Some STIs can also be transmitted through the blood or from the mother to the child during pregnancy or childbirth. Although in most cases STIs are asymptomatic, especially among women or at non-genital sites, the most common clinical manifestations include: ulcers, and genital or extragenital warts; vaginal, urethral or anorectal discharge; abdominal pain; dysuria and painful coitus.
STIs can cause different clinical syndromes and serious complications.
Some STIs, particularly those causing ulcers, such as syphilis and genital herpes, increase the risk of contracting or transmitting HIV infection. The physical, psychological and social consequences of STIs significantly worsen the quality of life of the people affected.
According to World Health Organisation (WHO) data, worldwide, over one million people contract an STI every day. Syphilis during pregnancy causes more than 300,000 foetal and neonatal deaths in the world each year. Over 290 million women are infected with the human papilloma virus (HPV), which causes more than 500,000 cases of cervical cancer and about 266,000 deaths per year. Over 500 million people are carriers of the herpes simplex virus type 2 (HSV-2) which causes genital herpes.1,2
In recent years in developed countries, a persistent increase in the incidence of STIs has been detected in men who have sex with men (MSM).3,4 In Spain, since 2000, there has been a rising trend in the number of gonococcal infections. Cases of syphilis have remained at consistently high levels since 2011. Among the under-25s, Chlamydia trachomatis infection was the most common STI reported, and lymphogranuloma venereum the least common.5
Medical history in the high-risk patient with suspected STIIntroduction: epidemiological data help identify the population groups most affected by STIs in each country. In general, in developed countries, the highest incidence of STI is detected in MSM, in people who use drugs and in transgender male and female sex workers.2,4,6–8
Medical historyTo carry out a full STI risk assessment for each individual, we need their complete medical history, which includes sociodemographic, clinical and behavioural variables. The patient should be interviewed alone and we need to create a suitable atmosphere of trust, guaranteeing confidentiality and explaining the reasons for asking about sexual practices. Questions should be concise, open and respectful, without making assumptions about sexual practices and avoiding judging the person.9
The patient should first of all be asked about the reason for consulting: specific symptoms of STI, whether genital (urethral/vaginal discharge), non-genital (anorectal, oropharyngeal, skin-related) or nonspecific (fever, malaise, weight loss, etc.); date of onset of symptoms; and time since he/she last had unprotected sex.4,10
In many cases, STIs are asymptomatic and do not even show clinical signs. In addition to a physical examination, it is therefore essential to identify sexual practices and the pattern of condom use. This helps us determine the individual risk for each patient, make an effective early clinical diagnosis of the STI and provide personalised advice on prevention.
Using a structured questionnaire is highly recommended, including at least the five variables recommended for assessing the risk of STI, the “five P” rule: Partners, Sexual Practices, Prevention of Pregnancy, Protection against STIs and Past STIs.4
Medical history: STI risk assessment, the “five P” rulePartners, Practices, Prevention of Pregnancy, Protection against STIs, Past STIs
Partners:
- •
Who are you having sex with, men, women or transgender people?
- •
How many sexual partners have you had in the last two months?
- •
How many sexual partners have you had in the last 12 months?
- •
Do you think any of your sexual partners in the last 12 months might have had sex with anybody else?
Practices:
- •
Have you had vaginal sex?
- •
Have you had insertive or receptive anal sex?
- •
Have you had oral sex?
- •
If the answer is yes, “Do you use condoms: never, sometimes or always?”
Prevention of pregnancy:
- •
What methods of contraception do you use?
Protection against STIs:
- •
How do you protect yourself from STIs and HIV?
Past history of STIs:
- •
Have you ever had an STI?
- •
Have any of your partners ever had an STI?
Additional questions to identify HIV and viral hepatitis risk include:
- •
Have you ever injected drugs?
- •
Have you ever exchanged sex for money or drugs?
- •
Have you ever shared sex toys?
- 1)
Sociodemographic variables: gender, age, country of origin, level of education.
- 2)
Behaviour indicators:
- •
Sexual partners: age of first sexual relations; number of sexual partners in the last year and throughout life; gender of sexual partners (male, female, transgender); practices prostitution or is a client of prostitutes.
- •
Sexual practices: type of sex (oral, anal, vaginal); use of condoms in each activity; time since last unprotected sex; shared use of sex toys, fisting and use of apps to find sexual partners.
- •
Use of substances of abuse: consumption of excess alcohol and/or drugs for sex; unprotected sexual practices under the effect of said substances; routes of drug administration (oral, nasal, parenteral, etc.); shared use of material for drug use; and participation in chemsex sessions.11
- •
- 3)
Clinical indicators: known drug allergies; STI history; vaccinations; other relevant medical history; and concomitant medication.
In both symptomatic and asymptomatic patients, a full clinical examination should be carried out, with special emphasis on the patient's symptoms and sexual practices. Particular attention should be paid to internal genitalia (vagina, cervix) and external genitalia (vulva, penis, testicles), oropharynx, perianal and rectal region, skin and lymphadenopathy.
- 1)
Skin and mucous membranes: they should be examined for lesions compatible with primary or secondary syphilis or other skin/mucous membrane manifestations associated with HIV infection or other STIs.
- 2)
Genital: inspection of external genitalia (vulva, penis, testicles). Characteristics of urethral or vaginal discharge: purulent, haemorrhagic, mucous. Palpation of the testicles and epididymis, considering size, shape and sensitivity. Cervical/vaginal examination with speculum, assessing the characteristics of vaginal discharge, cervical erythema or secretions and the presence of any lesions.
- 3)
Anal/rectal: external inspection of the anal region, observing for possible HPV, herpes, syphilis or chlamydia lesions. Internal assessment: proctoscopy should be performed with anal speculum and cold light lamp to assess for secretions and lesions in the rectal mucosa. Visualisation of the anal canal while extracting the speculum.
- 4)
Lymphadenopathy: axillary, inguinal, lateral/cervical, submandibular and supraclavicular.
Depending on the clinical symptoms, exploratory signs, sexual practices and any sharing of sex toys or materials for drug use, the following should be requested: serology for syphilis, HIV, HAV, HBV, HCV, urethral, cervical/vaginal, rectal or pharyngeal secretions, study of genital or extragenital ulcers and anal and/or cervical cytology with HPV detection.
In summary, to ensure effective early diagnosis and the correct treatment for the STI, diagnostic tests need to be guided by the clinical and behavioural indicators of each individual. Additionally in patients diagnosed with an STI, screening for other STIs is recommended, along with tracing of sexual contacts in the previous few months, in order to interrupt the chain of transmission and prevent reinfection of the patient.
Role of rapid tests in the diagnosis of STIs in high-risk patientsRationaleThe use of suitable microbiological tests is key to effective management of STIs. They are necessary for the diagnosis itself, screening in high-risk groups, monitoring of treatment, epidemiological surveillance, the study of antibiotic sensitivity, and research.
The spectrum of diagnostic tests in microbiology used in STI ranges from direct microscopy, culture, antigen detection and serology, to the detection of genomic material. Apart from culture, all of the above could be considered as rapid diagnostic tests. Significant advances have been made in the different methods, primarily in the area of molecular biology (MB), providing greater efficiency and a shorter response time.
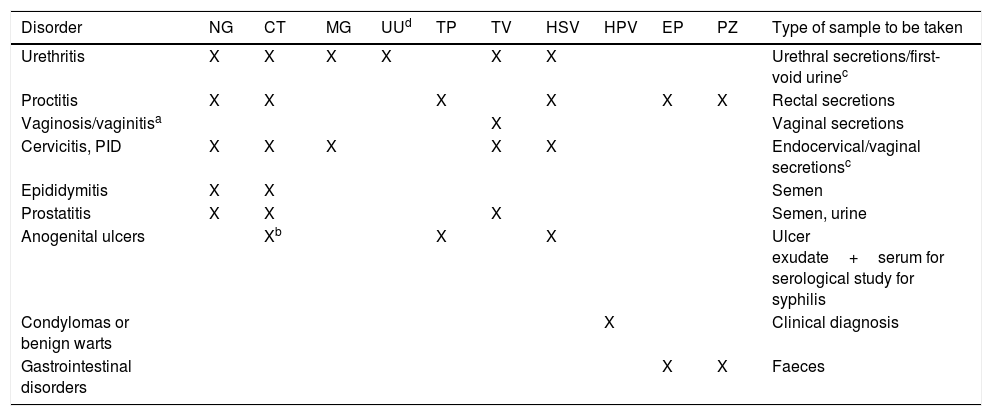
Sample collectionThe collection, transport and processing of samples are critical factors in the diagnostic effectiveness of microbiological techniques. The type of samples needed will depend on sexual practices. A genital sample should always be taken, and oropharyngeal and/or rectal swabs if the patient has had oral and/or anal sex. Table 1 shows the best sample to collect according to the aetiology and signs and symptoms.
Aetiological agents of the STI according to disorder and type of sample.
| Disorder | NG | CT | MG | UUd | TP | TV | HSV | HPV | EP | PZ | Type of sample to be taken |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urethritis | X | X | X | X | X | X | Urethral secretions/first-void urinec | ||||
| Proctitis | X | X | X | X | X | X | Rectal secretions | ||||
| Vaginosis/vaginitisa | X | Vaginal secretions | |||||||||
| Cervicitis, PID | X | X | X | X | X | Endocervical/vaginal secretionsc | |||||
| Epididymitis | X | X | Semen | ||||||||
| Prostatitis | X | X | X | Semen, urine | |||||||
| Anogenital ulcers | Xb | X | X | Ulcer exudate+serum for serological study for syphilis | |||||||
| Condylomas or benign warts | X | Clinical diagnosis | |||||||||
| Gastrointestinal disorders | X | X | Faeces |
CT: Chlamydia trachomatis; EP: enteropathogenic bacteria; HPV: human papillomavirus; HSV: herpes simplex virus 1 and 2; MG: Mycoplasma genitalium; NG: Neisseria gonorrhoeae; PID: pelvic inflammatory disease; PZ: intestinal protozoa; TP: Treponema pallidum; TV: Trichomonas vaginalis; UU: Ureaplasma urealyticum.
Bacterial vaginosis and Candida sp. are causes of vaginitis/vaginosis but are not considered to be STIs, despite the fact that they are related to sex.
The recommended samples are urethral secretions, in the case of urethritis, and endocervical secretions, in the case of cervicitis, PID and disorders associated with childbirth. For the molecular biology study, urine samples, in the case of urethritis, and vaginal secretions in the other instances have the same diagnostic efficiency if urethral or endocervical secretions respectively cannot be collected, while being less invasive.
Ureaplasma urealyticum has been linked to urethritis in men, especially when there is no other pathogen to explain the signs and symptoms. In women, it is considered as vaginal flora, although in some cases, as happens with Mycoplasma hominis, it has been linked to the threat of premature birth.
eSymptoms in the context of possible risky sexual practices.
Samples should be taken before starting antibiotic therapy and avoiding contact with disinfectants and antiseptics. They should be collected in the appropriate container, in a sufficient volume according to the test to be requested, and suitably identified. The techniques can give false negative results if sample collection and transport guidelines are not followed.12
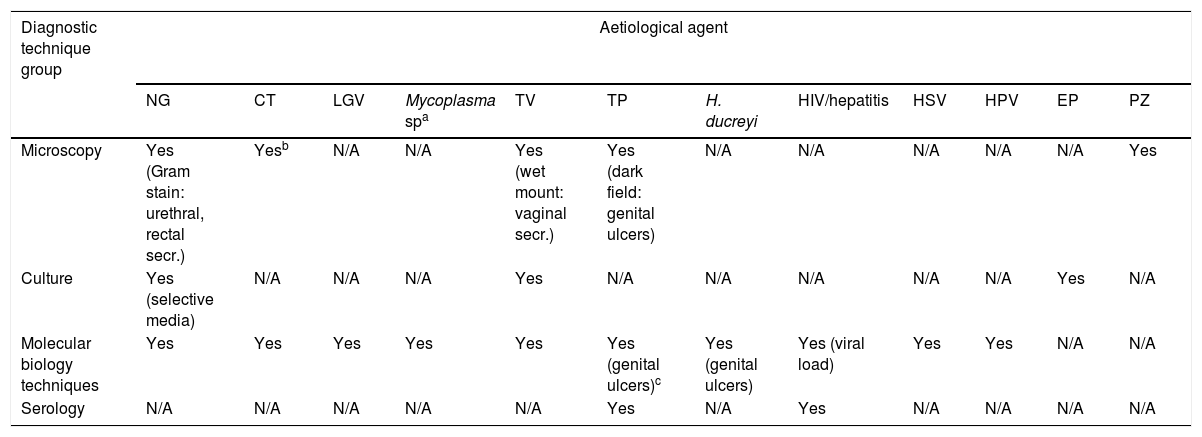
Types of microbiological diagnostic testThe most effective diagnostic techniques for studying the different microorganisms involved in STIs are shown in Table 2.
STI diagnostic techniques according to aetiological agent.
| Diagnostic technique group | Aetiological agent | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NG | CT | LGV | Mycoplasma spa | TV | TP | H. ducreyi | HIV/hepatitis | HSV | HPV | EP | PZ | |
| Microscopy | Yes (Gram stain: urethral, rectal secr.) | Yesb | N/A | N/A | Yes (wet mount: vaginal secr.) | Yes (dark field: genital ulcers) | N/A | N/A | N/A | N/A | N/A | Yes |
| Culture | Yes (selective media) | N/A | N/A | N/A | Yes | N/A | N/A | N/A | N/A | N/A | Yes | N/A |
| Molecular biology techniques | Yes | Yes | Yes | Yes | Yes | Yes (genital ulcers)c | Yes (genital ulcers) | Yes (viral load) | Yes | Yes | N/A | N/A |
| Serology | N/A | N/A | N/A | N/A | N/A | Yes | N/A | Yes | N/A | N/A | N/A | N/A |
CT: Chlamydia trachomatis; EP: enteropathogenic bacteria; LGV: lymphogranuloma venereum; N/A: not applicable; NG: Neisseria gonorrhoeae; PZ: intestinal protozoa; TP: Treponema pallidum; TV: Trichomonas vaginalis; HSV: herpes simplex virus 1 and 2; HPV: human papillomavirus.
Includes the aetiological agents Mycoplasma genitalium, Mycoplasma hominis and Ureaplasma urealyticum.
Microscopy techniques are a quick, simple and economical but observer-dependent tool. In the case of Neisseria gonorrhoeae (N. gonorrhoeae), Gram staining has high sensitivity (≥95%) and specificity in the diagnosis of symptomatic urethritis in men, but lower for other sites (endocervical or rectal secretions) and is not recommended in pharyngeal secretions. Dark-field microscopy examination of genital chancres provides an immediate result for the diagnosis of syphilis, but there are some limitations: it has to be analysed immediately after collection and requires experience in microscopy to minimise false negative results. This test is not recommended in mouth ulcers because saprophytic spirochetes may be visualised. Wet mount microscopic examination for the detection of Trichomonas vaginalis (T. vaginalis) is easy, quick and low cost, but, although it does have a specificity of 98%, it has low, observer-dependent sensitivity (62–92%). Microscopic diagnosis is essential for other protozoa of intestinal origin (Giardia, Entamoeba sp.) in cases of gastrointestinal symptoms related to the type of sexual practices, in particular in the male homosexual population, in view of the fact that the route of transmission is faecal-oral.13
Culture is indicated fundamentally for the isolation of N. gonorrhoeae from genital samples using selective culture media (Thayer-Martin agar). It is the recommended technique because of its sensitivity, specificity and suitability for multiple types of samples. Sensitivity to antibiotics and epidemiological studies can also be determined with culture; important nowadays because of the worrying increase in resistance. Culture of T. vaginalis is easy and inexpensive to perform, although it does require an incubation time of several days.
The MB methods used in clinical microbiology laboratories for the study of STIs are mainly gene amplification techniques based on the polymerase chain reaction. They are rapid, automated tests with high sensitivity and specificity which can be used to detect virtually all microorganisms and to measure viral load (HIV and hepatitis). Some of the polymerase chain reaction techniques are able to detect several STI-causing microorganisms in the same reaction, as well as determine the mechanisms of resistance to certain antibiotics (Mycoplasma genitalium [M. genitalium] – macrolides and quinolones). As a result of technological advances, rapid, easy-to-use molecular diagnostic options are becoming commercially available. They are still expensive, but the speed in obtaining diagnoses probably makes up for the high cost.
Serology is the technique used in the screening and/or diagnosis of Treponema pallidum (T. pallidum), HIV and hepatitis virus (A, B and C) infections. In the case of syphilis, treponemal tests (determination of specific antibodies) are used for screening/diagnosis and non-treponemal tests (determination of non-specific antibodies) to determine the status of the infection (active or inactive) and treatment follow-up. In all cases, there is evidence that immunochromatography (HIV, syphilis and hepatitis) and agglutination (RPR syphilis) are very useful for a rapid diagnosis.
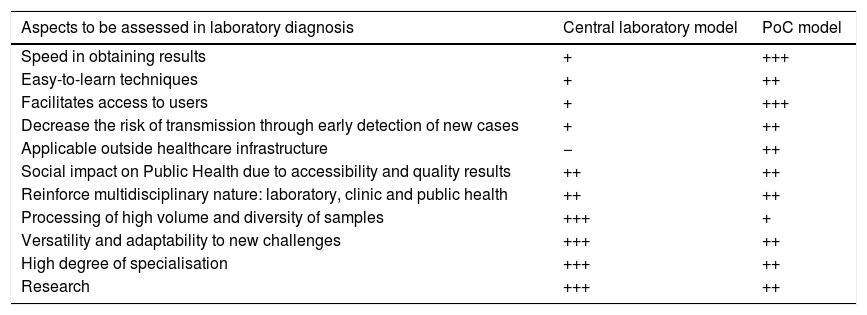
Rapid – “point of care” – microbiological diagnosisThere is a need for microbiological diagnostics to be decentralised to a certain extent nowadays in order to provide a faster response and allow earlier, targeted treatment. The WHO defines rapid or “point of care” (PoC) diagnostic tests as those which are: “Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free and Deliverable to end-users” (ASSURED).
The management of STIs is made easier and more effective by access to a rapid diagnosis, as the patient can often be dealt with in a single consultation.14 The rapid tests also enable patients to receive early, specific treatment, avoiding unnecessary and inadequate antibiotic therapy and minimising the chain of transmission, which helps prevent new cases. Qualitative studies also affirm the need for diagnostic tests in PoC format perceived by the end users and healthcare professionals.15
Moreover, the increase in the incidence of STIs is saturating the classic healthcare dynamic, making it necessary for us to adapt to a new scenario. The emergence of new rapid access circuits closer to users with self-collection of samples and PoC tests can complement diagnosis in centralised and specialised laboratories.16,17 These models have focused more on the screening of asymptomatic users, but may also be useful in cases with symptoms. Table 3 shows the potential benefits and drawbacks of diagnosis in hospital-based laboratories versus PoC centres.
Comparison of pros and cons of the centralised laboratory vs PoC diagnosis models.
| Aspects to be assessed in laboratory diagnosis | Central laboratory model | PoC model |
|---|---|---|
| Speed in obtaining results | + | +++ |
| Easy-to-learn techniques | + | ++ |
| Facilitates access to users | + | +++ |
| Decrease the risk of transmission through early detection of new cases | + | ++ |
| Applicable outside healthcare infrastructure | − | ++ |
| Social impact on Public Health due to accessibility and quality results | ++ | ++ |
| Reinforce multidisciplinary nature: laboratory, clinic and public health | ++ | ++ |
| Processing of high volume and diversity of samples | +++ | + |
| Versatility and adaptability to new challenges | +++ | ++ |
| High degree of specialisation | +++ | ++ |
| Research | +++ | ++ |
PoC: “point of care”.
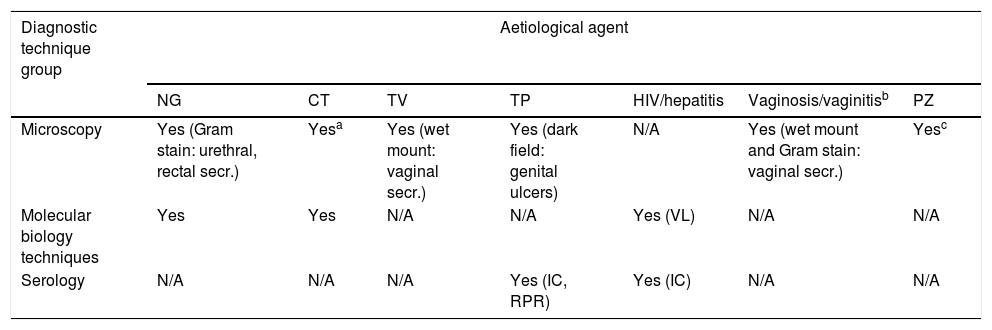
Of the tests mentioned in the previous section, we would consider microscopy, antigen/antibody detection and some MB techniques, to a greater or lesser degree, as suitable candidates for use as PoC tests. Table 4 shows a summary of the tests available by aetiological agent. All have the possibility of being implemented, in STI consultations or 24-h laboratories according to the context, to facilitate the diagnosis of STIs, whether in symptomatic cases or for screening at-risk populations.
Rapid PoC microbiological diagnostic techniques for STIs according to aetiological agent.
| Diagnostic technique group | Aetiological agent | ||||||
|---|---|---|---|---|---|---|---|
| NG | CT | TV | TP | HIV/hepatitis | Vaginosis/vaginitisb | PZ | |
| Microscopy | Yes (Gram stain: urethral, rectal secr.) | Yesa | Yes (wet mount: vaginal secr.) | Yes (dark field: genital ulcers) | N/A | Yes (wet mount and Gram stain: vaginal secr.) | Yesc |
| Molecular biology techniques | Yes | Yes | N/A | N/A | Yes (VL) | N/A | N/A |
| Serology | N/A | N/A | N/A | Yes (IC, RPR) | Yes (IC) | N/A | N/A |
CT: Chlamydia trachomatis; IC: immunochromatography; N/A: not applicable; NG: Neisseria gonorrhoeae; PZ: intestinal protozoa; RPR: rapid plasma reagin; TP: Treponema pallidum; TV: Trichomonas vaginalis; VL: viral load.
Microscopic study of urine sediment or a Gram stain of urethral exudate suggestive of urethritis (>5leukocytes/field) could point to non-gonococcal urethritis.
Counselling interventions and behavioural approaches are elements of primary prevention against STIs, including HIV. These interventions include:
- •
Sex education before and after the STI and HIV tests.
- •
Advice on safer sex practices and harm reduction.
- •
Promotion of condom use in all types of sex.
- •
Interventions aimed at key population groups, including sex workers, MSM and people who use drugs.
- •
Counselling and sex education adapted to adolescents.
The systematic use of condoms is one of the most effective methods of protection against STIs, including HIV. In addition, health education can improve people's ability to recognise the symptoms of STIs, which increases the likelihood of them seeking healthcare and advising their sexual partners to do so too. Unfortunately, the lack of availability of adequate diagnostic tests and the entrenched widespread stigma surrounding STIs continue to hinder the control of these infections.
Specific sexual health services should assess each person's needs and risks and establish basic prevention advice and interventions through combined biomedical approaches, perform periodic screening for STIs/HIV and post-treatment follow-up, and carry out contact tracing. Immediate access to antiretroviral treatment is essential for patients diagnosed with HIV. An important objective is to promote harm and risk reduction for patients with problematic drug use and refer to mental health services according to the needs of each patient.
Frequency of STI screening in people at high riskHIV/STI screening includes serology and testing for chlamydia and gonococcus depending on the type of sexual practices. The serology tests should initially include HIV, syphilis, HBV and HCV. The regularity of the screening will depend on the individual risk assessment.
In Spain18, periodic STI/HIV screening is recommended for:
- •
Sexually active people belonging to groups with a high prevalence of STIs, young people under the age of 25, MSM, sex workers, adolescents and people in prison or other places of detention.
- •
Sexually active HIV-infected patients annually, or more often depending on the individual risk assessment.
- •
Pregnant women in the 1st trimester of pregnancy, and repeat before delivery according to the woman's situation and risk practices. In pregnant women who have not attended prenatal check-ups, perform at the time of delivery.
- •
Screening for HPV infection and ruling out cervical carcinoma in women with or without HIV is recommended.
- •
In recent years, a number of countries have introduced recommendations on the frequency of testing for HIV and other STIs based on behavioural indicators.
In the United States, the following screening tests are recommended:
- •
All adults and adolescents aged 13–64 should be tested for HIV at least once in their lifetime.
- •
Annual testing for chlamydia and gonorrhoea in all sexually active women under 25, and in older women with multiple sexual partners or with a sexual partner with an STI.
- •
Screening for syphilis, HIV, hepatitis B, chlamydia and gonorrhoea in all pregnant women.
- •
Screening for syphilis, chlamydia and gonorrhoea at least once a year in all sexually active men: heterosexual, bisexual and MSM. MSM with multiple partners should be screened for HIV/STIs more frequently, at intervals of three to six months.
- •
Anyone who has unprotected sex or shares material for injecting drugs should have an HIV test at least once.19,20
In the United Kingdom, screening for STIs at least once a year is recommended, or every three months for all people with high-risk behaviours.21
The advice in Australia is that, after assessing the patient's risk, STI screening tests should be offered up to four times a year to all MSM who fall into one or more of the following categories: anal sex without protection; more than 10 sexual partners every six months; participation in group sex; and use of drugs for sex. In HIV-positive patients, serology for syphilis, chlamydia and gonorrhoea should be performed whenever the CD4+ T lymphocyte count and HIV viral load are determined.22
Summary of the preventive interventions for STIs4,7,23
- 1)
Sex and drug education.
- 2)
Promotion of condom use.
- 3)
Periodic screening, rapid diagnosis and immediate treatment of STIs/HIV.
- 4)
Pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP) for HIV and antiretroviral “treatment as prevention” (TasP).
- 5)
Hepatitis A, B and C: prevention, screening, vaccination and treatment.
- 6)
Anal and cervical cancer screening. HPV vaccination.
- 7)
Specific preventive interventions aimed at people with problematic drug use. Referral to mental health services.
Treatment of STIs in people at high risk is especially important for a number of reasons. It reduces morbidity and mortality rates in both the short-term (e.g. chlamydial cervicitis and pelvic inflammatory disease) and the long-term (as may occur in HIV and late latent syphilis). Moreover, the treatment of both symptomatic and asymptomatic individuals helps shorten the period of transmissibility, preventing new infections.17 In the case of HIV, different studies have demonstrated that a sustained undetectable viral load in the blood (deriving from good adherence to an effective antiretroviral treatment) prevents sexual transmissibility of HIV (“undetectable=untransmittable”).24 In chronic-recurrent infections (genital herpes or warts due to HPV), treatment of recurrences and shortening of the period in which the affected person has lesions also reduces the risk of transmission, although it is not completely eliminated.25,26 It is advisable to combine pharmacological treatment with health education and contact tracing.
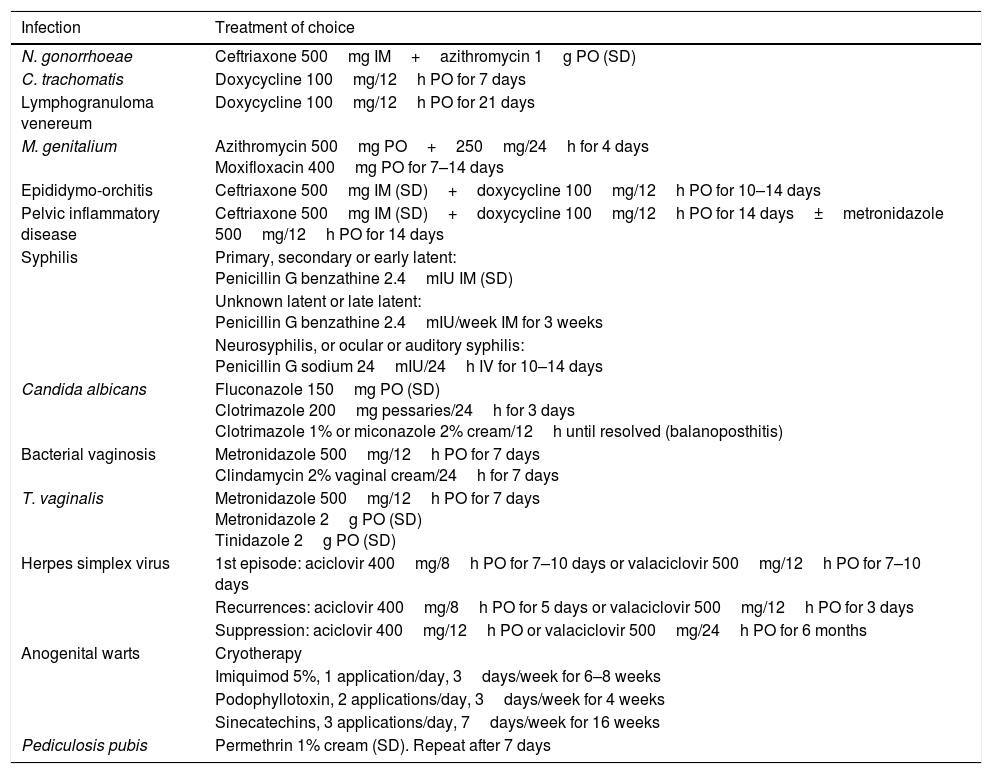
In general, there is no difference in the treatment of choice for STIs between people living with HIV and people without HIV. However, there are some exceptions. For example, in T. vaginalis infection, long treatment regimens are preferred instead of single doses, and in herpes simplex virus (HSV) infection, the doses for patients with HIV are higher than the usual doses.18Table 5 shows the preferred treatment regimens for the main STIs according to the latest versions of the clinical guidelines.4,10,18 More information will be available in each chapter about the preferred treatment and alternative therapies for the different STIs. This chapter will focus on the importance of assisted counselling, contact tracing and HIV prophylaxis before and after exposure to risk.
Treatment of choice for the main sexually transmitted infections and other related infections.
| Infection | Treatment of choice |
|---|---|
| N. gonorrhoeae | Ceftriaxone 500mg IM+azithromycin 1g PO (SD) |
| C. trachomatis | Doxycycline 100mg/12h PO for 7 days |
| Lymphogranuloma venereum | Doxycycline 100mg/12h PO for 21 days |
| M. genitalium | Azithromycin 500mg PO+250mg/24h for 4 days Moxifloxacin 400mg PO for 7–14 days |
| Epididymo-orchitis | Ceftriaxone 500mg IM (SD)+doxycycline 100mg/12h PO for 10–14 days |
| Pelvic inflammatory disease | Ceftriaxone 500mg IM (SD)+doxycycline 100mg/12h PO for 14 days±metronidazole 500mg/12h PO for 14 days |
| Syphilis | Primary, secondary or early latent: Penicillin G benzathine 2.4mIU IM (SD) |
| Unknown latent or late latent: Penicillin G benzathine 2.4mIU/week IM for 3 weeks | |
| Neurosyphilis, or ocular or auditory syphilis: Penicillin G sodium 24mIU/24h IV for 10–14 days | |
| Candida albicans | Fluconazole 150mg PO (SD) Clotrimazole 200mg pessaries/24h for 3 days Clotrimazole 1% or miconazole 2% cream/12h until resolved (balanoposthitis) |
| Bacterial vaginosis | Metronidazole 500mg/12h PO for 7 days Clindamycin 2% vaginal cream/24h for 7 days |
| T. vaginalis | Metronidazole 500mg/12h PO for 7 days Metronidazole 2g PO (SD) Tinidazole 2g PO (SD) |
| Herpes simplex virus | 1st episode: aciclovir 400mg/8h PO for 7–10 days or valaciclovir 500mg/12h PO for 7–10 days |
| Recurrences: aciclovir 400mg/8h PO for 5 days or valaciclovir 500mg/12h PO for 3 days | |
| Suppression: aciclovir 400mg/12h PO or valaciclovir 500mg/24h PO for 6 months | |
| Anogenital warts | Cryotherapy |
| Imiquimod 5%, 1 application/day, 3days/week for 6–8 weeks | |
| Podophyllotoxin, 2 applications/day, 3days/week for 4 weeks | |
| Sinecatechins, 3 applications/day, 7days/week for 16 weeks | |
| Pediculosis pubis | Permethrin 1% cream (SD). Repeat after 7 days |
IM: intramuscular; IV: intravenous; PO: oral; SD: single dose.
Being diagnosed with a sexually transmitted infection can provoke a great deal of stress and generate anxiety. One of a doctor's jobs is to advise and inform the patient. They therefore need to provide information about the infection, while providing reassurance to reduce the patients unease and ensure good adherence to treatment. After giving the diagnosis, the doctor needs to inform the patient about the nature of the aetiological agent, possible methods of transmission (risk associated with each method) and the natural history of the infection if not treated. Additionally, to ensure good adherence to treatment, it is best to inform the patient about both the efficacy of the treatment and any adverse effects that may occur while they are taking it. The importance of refraining from sex (abstinence) during the treatment should also be stressed to avoid further transmission to sexual partners. Lastly, the opportunity should be taken at each medical consultation for an STI to emphasise the different STI prevention measures, and allow sufficient time to resolve any uncertainties the patient may have.18
Contact tracing (CT) is defined as the identification and tracing of sexual partners (“contacts”) of a person diagnosed with an STI (“index case”) to provide them with information and an early diagnosis and treatment (if required). Treating contacts prior to confirming the infection with laboratory tests is known as “epidemiological treatment”.27 In general terms, and in the absence of medical contraindication, the regime for epidemiological treatment is simply the treatment of choice for each STI.
CT is not mandatory and must be carried out in compliance with EU laws and in accordance with the declaration of human rights. The most common reasons for the index case refusing to become involved in CT are being scared, worrying about loss of confidentiality and failure to accept the diagnosis. Therefore, the healthcare professional responsible for the CT must be able to show empathy and create a confidential, trusting atmosphere in order to carry the process through.
CT has many aims and benefits for both the index case and the contacts:
- •
Interruption of the chain of transmission.
- •
Prevention of reinfection of the index case by their sexual partners in the case of curable STIs.
- •
Increased diagnosis of asymptomatic infections, preventing the development of symptoms or long-term complications.
CT also helps bring new users to STI clinics, creating the opportunity to further promote prevention measures, provide more people with sex and health education and carry out screening for other STIs. It also has benefits for public health, by shortening the period of transmissibility among contacts and reducing asymptomatic cases in the general population.
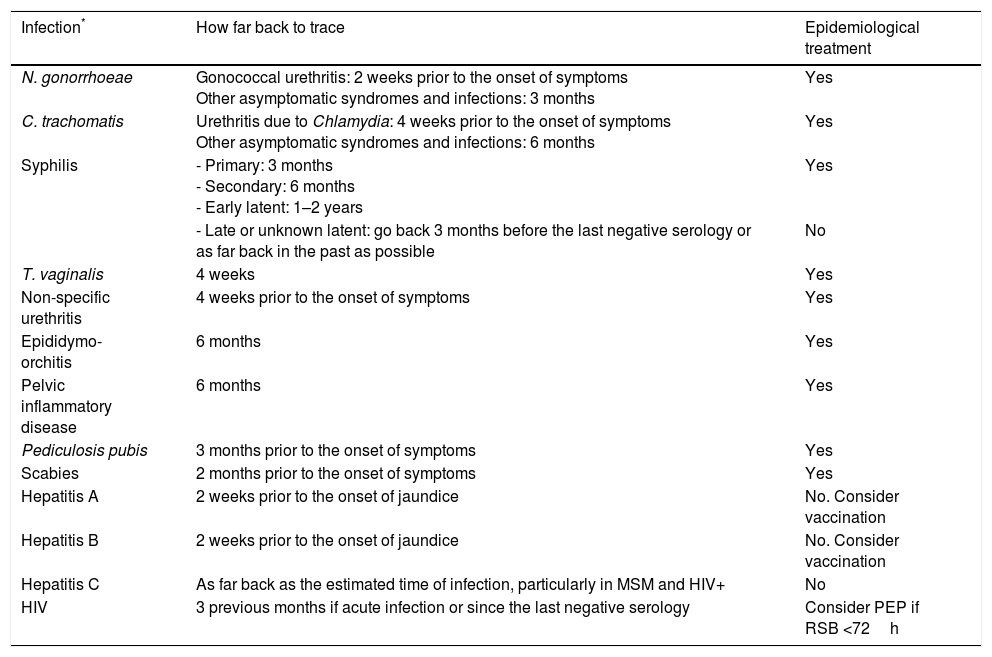
How far back to trace contacts depends on each STI. Table 6 sets out information for the main STIs in which CT is recommended along with the need for epidemiological treatment.
Contact tracing. How far back to trace contacts according to the most prevalent STIs and requirement for epidemiological treatment.
| Infection* | How far back to trace | Epidemiological treatment |
|---|---|---|
| N. gonorrhoeae | Gonococcal urethritis: 2 weeks prior to the onset of symptoms Other asymptomatic syndromes and infections: 3 months | Yes |
| C. trachomatis | Urethritis due to Chlamydia: 4 weeks prior to the onset of symptoms Other asymptomatic syndromes and infections: 6 months | Yes |
| Syphilis | - Primary: 3 months - Secondary: 6 months - Early latent: 1–2 years | Yes |
| - Late or unknown latent: go back 3 months before the last negative serology or as far back in the past as possible | No | |
| T. vaginalis | 4 weeks | Yes |
| Non-specific urethritis | 4 weeks prior to the onset of symptoms | Yes |
| Epididymo-orchitis | 6 months | Yes |
| Pelvic inflammatory disease | 6 months | Yes |
| Pediculosis pubis | 3 months prior to the onset of symptoms | Yes |
| Scabies | 2 months prior to the onset of symptoms | Yes |
| Hepatitis A | 2 weeks prior to the onset of jaundice | No. Consider vaccination |
| Hepatitis B | 2 weeks prior to the onset of jaundice | No. Consider vaccination |
| Hepatitis C | As far back as the estimated time of infection, particularly in MSM and HIV+ | No |
| HIV | 3 previous months if acute infection or since the last negative serology | Consider PEP if RSB <72h |
MSM: men who have sex with men; PEP: post-exposure prophylaxis; RSB: risky sexual behaviour.
Despite international efforts and preventative measures already in place, there has not been a significant decrease in new cases of HIV in the last decade. In 2017, there were 1,800,000 new infections worldwide.28 In the West, figures are particularly significant among MSM and transgender women, with these groups now having the highest rates of new infections (53% of new infections in the EU/EEA in 2016 for which the method of transmission was known were in MSM).29 A new approach to prevention has therefore been required to try to make progress towards eradicating the epidemic.
PrEP prophylaxis is an additional HIV prevention measure, complementary to all the other measures used up to now (condom use, population screening and early diagnosis, health education, undetectability of people living with HIV). PrEP consists of the administration of emtricitabine (FTC) 200mg/tenofovir (TDF) 300mg in a single tablet to people who are HIV-negative but at high risk of acquiring HIV. The prophylaxis is given as part of a comprehensive programme including periodic STI and HIV screening, ongoing medical advice and health education and promotion of other preventive measures, without which the efficacy of the prophylaxis would be reduced.
The recommendations in Spain for PrEP have been established for individuals whose characteristics mean they belong to a high-risk group with an HIV incidence of two cases/100 person-years or higher; falling into this category are MSM and transgender women who also meet any of the following criteria30:
- •
Multiple sexual partners
- •
Anal sex without a condom
- •
Recreational substance abuse
- •
Diagnosis of a bacterial STI
- •
Having required post-exposure prophylaxis.
The effectiveness of PrEP has been demonstrated in numerous different clinical trials.30 In the Ipergay and PROUD studies conducted in MSM, an 86% reduction was found in the risk of acquiring HIV. In later studies such as the follow-up cohort of the Ipergay study, even higher effectiveness was demonstrated, with a 97% risk reduction. The high efficacy of this measure has been found to be closely linked to adherence. In the cohort study conducted with the users previously included in the ATN 082, iPrex and US Safety Study trials, in users with adherence levels of four or more tablets per week, the incidence of HIV was found to be 0/100 person-years.31 There are two ways to take PrEP: one tablet a day; or dosing related to having sex, consisting of taking two tablets before exposure and then one tablet a day for two days after the last exposure.
The continued use of FTC/TDF can lead to various adverse effects, although they are reversible. Self-limiting nausea and gastrointestinal discomfort has been reported in clinical trials in the first few weeks. The use of tenofovir disoproxil fumarate (TDF) is associated with renal toxicity and decreased bone mineral density, although these disorders are reversible. The introduction of PrEP has raised concerns about compensatory behavioural changes (increased high-risk sex) in the population and a consequent increase in STIs. In the studies carried out to date, although the incidence has been high, which is not surprising given the high-risk criteria of the users, there has been no significant increase in the number of STIs.32,33
Post-exposure prophylaxisPost-exposure prophylaxis (PEP) after risky sexual behaviour (RSB) consists of the administration of antiretroviral therapy (ART) at an early stage for a limited period of 28 days to prevent HIV infection. There are few efficacy data regarding PEP as, for ethical reasons, it is not feasible to carry out clinical trials. However, PEP efficacy has been demonstrated in animal studies and observational studies.34
For PEP to be adequately prescribed, the risk after sexual exposure has to first be assessed and will be affected by multiple factors:
- -
Sexual practices: Receptive anal sex without a condom is considered to be the practice with greatest risk, followed by insertive anal sex, receptive vaginal sex and insertive vaginal sex. Oral sex is considered to be low or minimal risk.35
- -
Serological status of the source. If the source is known to be HIV+, a high viral load increases the chances of infection, while people with undetectable viral load do not transmit HIV. We also have to take into account the presence of resistance mutations in the virus which may result in modification of the ART regimen. Where HIV status is unknown, the possibility of the source belonging to a population with a higher prevalence of HIV has to be taken into account. Having an STI also increases the risk.
- -
Host susceptibility. The presence of breaks in the genital mucosa due to STI, traumatic sex or host disease can enhance the risk of acquiring HIV. Circumcision decreases the likelihood in heterosexual males.
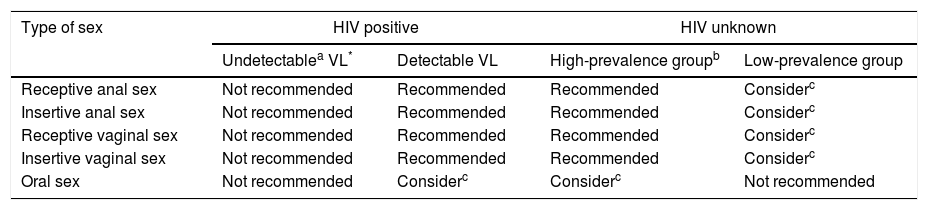
The risk of infection can be calculated by multiplying the risk of the source being HIV+ (from the estimated prevalence) by the risk according to sexual practices. PEP is indicated at risks greater than 1/1000 and should be considered when risk is from 1/1000 to 1/10,000.35Table 7 shows a summary of the recommendations for the administration of PEP, although the indication should always be agreed with the user once risks and benefits have been assessed.
Recommendations for the indication of post-exposure prophylaxis (PEP).
| Type of sex | HIV positive | HIV unknown | ||
|---|---|---|---|---|
| Undetectablea VL* | Detectable VL | High-prevalence groupb | Low-prevalence group | |
| Receptive anal sex | Not recommended | Recommended | Recommended | Considerc |
| Insertive anal sex | Not recommended | Recommended | Recommended | Considerc |
| Receptive vaginal sex | Not recommended | Recommended | Recommended | Considerc |
| Insertive vaginal sex | Not recommended | Recommended | Recommended | Considerc |
| Oral sex | Not recommended | Considerc | Considerc | Not recommended |
Taking PEP is extremely stressful for users as they have to come to terms with the possibility that they may have been infected with HIV. Psychological support therefore needs to be provided, and the importance of adherence to ART and medical follow-up should be emphasised. It is also an opportunity to provide sex education and assess the indication for PrEP.
The ART regimen indicated at present is the combination of two NRTI (emtricitabine/tenofovir) and an integrase inhibitor. This regimen has been shown to have good tolerance and few adverse effects. However, a good medical history must be taken to avoid interactions or undesirable effects. For example, in the case of elvitegravir, combination with cobicistat (cytochrome P450 inhibitor) can lead to interactions with concomitant medication or the use of recreational substances.36
PEP has to be started within the first 72h of exposure and ideally within the first 24h. The established duration is 28 days. A general analysis (complete blood count and basic biochemistry) should be carried out at the beginning as well as STI screening. It is recommended that screening for STI and HIV serology be repeated at 4 and 12 weeks. In the event of HIV infection, the user should be referred to a specialist to continue the treatment and perform a full assessment.
Conclusions- -
In recent years, a persistent increase in the incidence of STIs has been detected in Spain, predominantly affecting men.
- -
Rapid microbiological diagnosis of STIs and immediate treatment are the best way to break the chain of transmission.
- -
The preventive harm reduction interventions for STIs need to be adapted and orientated to the sociodemographic and behavioural characteristics of the most affected populations.
- -
In adolescents, HPV vaccine coverage has to be increased.
- -
Of great benefit would be to update, adapt, disseminate and generalise the use of all STI/HIV preventive measures which have been shown to be effective.
- -
Easily accessible STI clinics are the ideal mechanisms for rapid, early diagnosis and immediate treatment of STIs.
- -
Accurate clinical and microbiological diagnoses are essential for the best clinical-epidemiological management of STIs.
- -
Optimal efficiency in terms of microbiological diagnosis depends on full awareness of the possible aetiological agents according to the signs and symptoms, which determines the type of samples to be collected and tests to be requested.
- -
Molecular biology diagnostic techniques have helped improve the aetiological diagnosis of STIs, providing greater sensitivity and reducing response times.
- -
The implementation of rapid response microbiological diagnostic systems enables early treatment to interrupt the chain of transmission and prevent major clinical complications.
- -
The emergence of resistance to antibiotics (M. genitalium and N. gonorrhoeae) requires epidemiological surveillance of antimicrobial sensitivity.
- -
We have to be aware of other germs, such as parasites and enteropathogenic bacteria, which do not cause actual genital disease, but can be transmitted sexually.
- -
CT is defined as the identification and tracing of sexual partners (“contacts”) of a person diagnosed with an STI (“index case”).
- -
CT is not mandatory and must be carried out in compliance with EU laws and in accordance with the declaration of human rights.
- -
How far back to trace contacts and the need for epidemiological treatment depends on the STI diagnosed.
- -
PrEP prophylaxis is an additional HIV prevention measure, complementary to all the other measures used up to now.
- -
PrEP consists of the administration of emtricitabine (FTC) 200mg/tenofovir (TDF) 300mg in a single tablet, accompanied by periodic STI and HIV screening, ongoing medical advice and health education and promotion of other preventive measures.
- -
PrEP is indicated in MSM and transgender women at high risk of HIV infection.
- -
Given the likelihood of tenofovir-related renal toxicity, periodic monitoring of renal function is recommended in PrEP users.
- -
PEP after RSB consists of the early administration of ART (first 72h) for a limited period of 28 days to prevent HIV infection.
- -
For PEP to be adequately prescribed, the risk after sexual exposure has to first be assessed, taking multiple factors into account associated with sexual practices, the sexual partner and users themselves.
- -
Psychological support needs to be provided during the PEP, and the importance of adherence to ART and medical follow-up emphasised. It is also an opportunity to provide sex education and assess the indication for PrEP.
The Sociedad Española de Infecciones y Microbiología Clínica (SEIMC) [Spanish Society of Infections and Clinical Microbiology] has paid the authors of this manuscript for the time they dedicated to this work in accordance with applicable regulations.
Conflicts of interestThe authors declare that they have no conflicts of interest.
To Carmen Rodríguez Martín◊, Mar Vera García◊, Teresa Puerta López◊ and Oskar Ayerdi Aguirrebengoa◊, Juliana Esperalba Esquerra, Judit Serra-Pladevall◊ and Paula Salmerón Menéndez.
◊ Member of the SEIMC Grupo de Estudio de Infecciones de Transmisión Sexual (GEITS) [STI Study Group].
Please cite this article as: del Romero J, García-Pérez JN, Espasa-Soley M. Prevención y tratamiento de las infecciones de transmisión sexual en personas con alto riesgo, incluyendo pacientes infectados por el VIH. Enferm Infecc Microbiol Clin. 2019;37:117–126.